Cornopsylla magna, Luo, Xinyu, Li, Qiang, Li, Fasheng & Cai, Wanzhi, 2013
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3646.2.2 |
|
publication LSID |
lsid:zoobank.org:pub:E28E6352-2AD5-432E-BC58-B3A345E266EA |
|
DOI |
https://doi.org/10.5281/zenodo.6159912 |
|
persistent identifier |
https://treatment.plazi.org/id/733487C2-FFDC-FF9B-4AEF-0A9C6584FF20 |
|
treatment provided by |
Plazi |
|
scientific name |
Cornopsylla magna |
| status |
sp. nov. |
Cornopsylla magna View in CoL sp. nov.
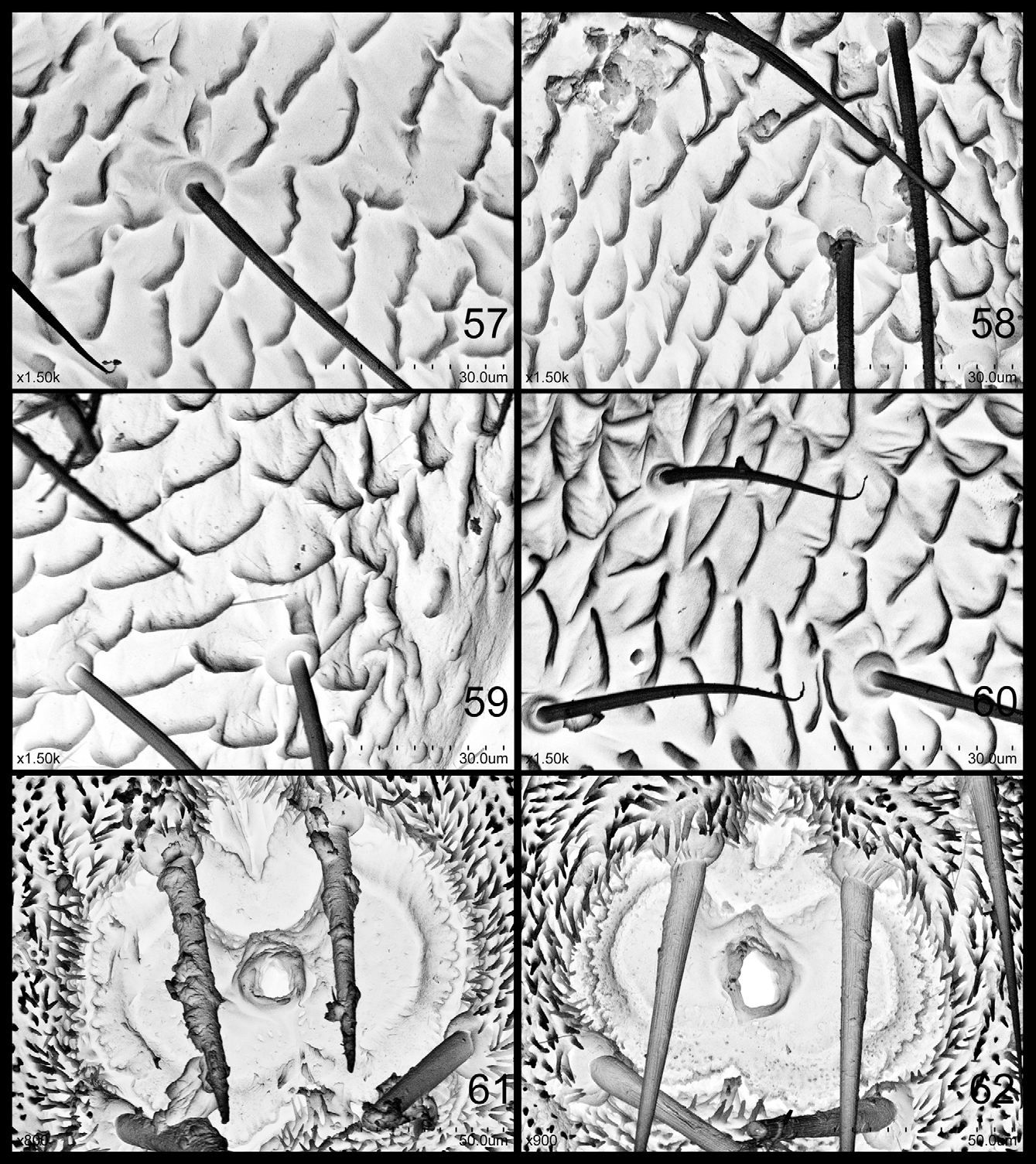
( Figs 23–33 View FIGURES 23 – 30 View FIGURES 31 – 33 , 58, 61 View FIGURES 57 – 62 )
Adult. Coloration: Body green in general. Vertex light green, discal foveae and surrounding small area light orange. Genal process light green. Compound eyes black, ocelli orange. Antenna green, with apical 1/4 of segment III, apical half of IV, apical 2/3 of V, VI and VII black, and segments VIII–X entirely black. Mesopraescutum and mesoscutum with yellowish brown patterns/stripes. Legs green. Fore wing hyaline and clear, veins brown. Apical tooth of paramere black. Female terminalia yellow, area in proctiger surrounding lateral and posterior (basal) margin of anus black, apical process of proctiger and apical half of subgenital plate black.
Structures: Body relatively robust and hairy. Head ( Fig. 23 View FIGURES 23 – 30 ) large, conspicuously wider than mesoscutum, inclined from longitudinal body axis by about 60°–70°. Genal processes ( Fig. 23 View FIGURES 23 – 30 ) long-cone shaped, longer than vertex along median suture, moderately divergent with inner margin nearly straight and outer margin curved outwards; apex subacute, genal whip setae conspicuously longer than normal setae. Metatibia without basal spine, apical spurs typically 7 and arranged as (1+4+1+1), with median 5 randomly grouped sometimes. Fore wing ( Fig. 30 View FIGURES 23 – 30 ) oblong oval, widest at apical 1/3; pterostigma long and narrow, smoothly transiting into vein R1; pterostigma, C+Sc, R1, R+M+Cu1, R, base of Rs and A1+2 with extraordinary long setae, gradually turning into normal tiny pterogostic setae apically; surface spinules present in all cells, leaving wide spinule-free bands along veins; radular spinules present in cells cu1, m2 and m1, in r2 too dim to be told.
Male terminalia: Proctiger ( Fig. 24 View FIGURES 23 – 30 ) tubular and slightly curved, evenly covered with long setae. Paramere ( Figs 24 & 25 View FIGURES 23 – 30 ) in profile relatively broad, very shallowly rooted in subgenital plate; basal 1/7 broad, then steeply narrowed, subapex strongly widened and curving, making apex curved inward; apical tooth ( Fig. 26 View FIGURES 23 – 30 ) relatively small, with tip rounded and pointing cephalad, base slightly constricted; anterior margin of subapical widened section serrate and relatively well developed, with one strong seta growing from each “sawtooth”; outer surface with several setae inclining posteriorly in basal half, and evenly spaced setation in apical half; posterior margin with rather long setae in basal half; inner surface with several short setae subapically, and dense long setae in basal half; three short setae based in inner surface of apical tooth always present. Distal segment of aedeagus ( Fig. 27 View FIGURES 23 – 30 ) nearly straight; apical dilatation taking about 1/3 length of distal segment of aedeagus, smoothly transiting from the latter, with apex rounded; sclerotised end tube of ductus ejaculatorius curved caudad-dorsally, rising high beyond dorsal surface. Subgenital plate ( Fig. 24 View FIGURES 23 – 30 ) with one horizontal band of setae in dorsal margin and evenly spaced setae in ventral surface.
Female terminalia ( Fig. 28 View FIGURES 23 – 30 ): Bulging before apical process of proctiger relatively tall. Apical process of proctiger relatively thin and moderately rising upward, with dorsal surface slightly waved; setation in apical process of proctiger asymmetrical by longitudinal body axis, each seta in figure maybe absent. Subgenital plate wide and subglobular in basal half, abruptly shrinking in mid, then gradually attenuated apically; several long setae present in basal half of narrow part; membranous part anterior to base with one or two long setae.
Intraspecific variation: For dried specimens, females generally have darker coloration than males.
Fifth instar nymph. Coloration: For specimens preserved in absolute ethanol. Body yellow. All sclerites of head and thorax, free sclerites in abdomen light brown. Wing pads brown. Legs yellow. Compound eyes grey. Antenna yellow, with black apices on segments 4–7; segment 8 black except for basal 1/6. Caudal plate both ventrally and dorsally black. Ventral abdominal 2+2 large lateral free sclerites black, 4+4 median free sclerites brown.
Structures. Body margin expanded before fore wing pad, covering anterior 1/2 of the latter, gradually growing congruent with it ( Fig. 31 View FIGURES 31 – 33 ). Tarsal arolium ( Fig. 33 View FIGURES 31 – 33 ) relatively large and fan-shaped, anterior margin moderately depressed; lateral lobe relatively broad; unguitractor rather slender. Field anterior to circum anal pore field rising upward and strongly extending caudad, forming one “cave” with “roof” dehiscing, covering anterior half of pore field, with posterior margin of inner and outer circum anal pore rings and anus visible ( Fig. 32 View FIGURES 31 – 33 ). Outer circum anal pore ring ( Fig. 61 View FIGURES 57 – 62 ) complete, consisting of long-narrow suture-shaped pores; posterior margin slightly depressed, anterior margin abruptly depressed. Inner circum anal pore ring ( Fig. 61 View FIGURES 57 – 62 ) as a broad band of relatively scattered small ellipse pores; posterior margin complete and slightly depressed in middle, anterior margin broken in middle. 2+2 strong setae present anterior-lateral to anal pore field, the line connecting their roots nearly transversely straight ( Fig. 32 View FIGURES 31 – 33 ).
Materials examined. Holotype: Male, dry mounted, China, Yunnan, Yongshan, Jinzhai Village, 1453 m, on Zanthoxylum bungeanum , 29.vi.2012, Yin Shanshan.
Paratypes: 19 male, 22 female, and dozens of specimens preserved in absolute ethanol, with the same data as holotype. One series of adults and 5th-instar nymphs, preserved in absolute ethanol, China, Yunnan, Yongshan, Jinzhai Village, 1453 m, on Zanthoxylum bungeanum , 26.v.2012, Yin Shanshan.
Host plant: Zanthoxylum bungeanum .
Etymology. This species is named after its currently largest body size in this genus. “Magnus” = “large”.
Remarks. According to Miss Yin Shanshan (personal communication), who is working on the biology and population dynamics of this species for her master’s degree, this species started to “cause damage” (which we infer to mean incubation of the first generation) in late April 2011, and was most destructive from early May to early June and from early July to mid September. The nymphs aggregate on shoots and young leaves, not inducing galls, but causing leaf-rolling. They also prefer shade and staying still in rolled leaves. Honey dew and wax are also secreted. Detailed information on the biology of this species will be published by Yin Shanshan.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.