Cyrtodactylus chrysopylos Bauer, 2003
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4527.2.1 |
|
publication LSID |
lsid:zoobank.org:pub:18D79777-5B8B-4D57-8AF5-B19BE46E48AC |
|
DOI |
https://doi.org/10.5281/zenodo.6489084 |
|
persistent identifier |
https://treatment.plazi.org/id/7823879A-B56D-FFF6-FF2B-20ECE15BFD96 |
|
treatment provided by |
Plazi |
|
scientific name |
Cyrtodactylus chrysopylos Bauer, 2003 |
| status |
|
Cyrtodactylus chrysopylos Bauer, 2003
Nwalabo Mountain Cave Bent-toed Gecko
( Figs. 5 View FIGURE 5 , 7 View FIGURE 7 , 10 View FIGURE 10 )
Holotype. Adult male CAS 22641 collected on 14 July 2002 by G. O. U. Wogan, R. S. Lucas, J. V. Vindum, Htun Win, Thin Thin, Awan Khwi Shen, and H. Tun from “Panlaung-Pyadalin Cave Wildlife Sanctuary, Ywangan Township, [Taunggyi District], Shan State, Myanmar (21°0’58.4”N, 96°20’25.0”E)” at 306 m in elevation. The type locality “Panlaung-Pyadalin Cave Wildlife Sanctuary…” ( Bauer 2003) encompasses an area of 333.8 km 2 ranging from 118–1371 m in elevation. This region contains varying ecological regions along an elevational transect from dry lowland Indaing and mixed deciduous forests to dry and wet upper mixed deciduous forests (Grismer 2018). The topography of the area is equally varied and sculpted by limestone cliff faces and their associated rocky foothills and scree, shallow river basins, and narrow floodplains. Although no natural history data were provided with the description of C. chrysopylos , its coordinates place it at the Pyadalin Cave . Given that the seven additional specimens reported here were collected from within the Pyadalin Cave and are essentially genetically identical to the holotype, we restrict the type locality here to the Pyadalin Cave , Panlaung-Pyadalin Cave Wildlife Sanctuary , Ywangan Township, Shan State, Myanmar (21.13275°N, 96.34026°E; 303 m). GoogleMaps
Additional specimens examined here from the type locality. GoogleMaps LSUHC 13126–27 View Materials collected by Myint Kyaw Thura, L. Lee Grismer, Marta S. Grismer, and Matthew L. Murdoch on 24 March 2017 and LSUHC 13934–38 View Materials collected by Myint Kyaw Thura, L. Lee Grismer, Perry L. Wood, Jr., and Nyo Min Htwe GoogleMaps on 24 March 2018 from the Pyadalin Cave GoogleMaps within the Panlaung-Pyadalin Cave Wildlife Sanctuary GoogleMaps , Ywangan Township, Shan State, Myanmar ” (21.13275°N, 96.34026°E; 303 m).
Diagnosis. Cyrtodactylus chrysopylos differs from other species of Cyrtodactylus by having the unique combination of the following characters: 8–11 supralabials and infralabials; 30–35 paravertebral tubercles; 16–20 longitudinal rows of body tubercles; well-developed body tubercles extending past the hemipenial swellings; no gular tubercles; ventrolateral folds; 39–55 ventral scales; digits not relatively short; basal subdigital lamellae expanded proximal to digital inflection; 19–23 subdigital lamellae on the fourth toe; no enlarged femoral scales or pore-bearing femoral scales; no precloacal groove; 8–13 pore-bearing, contiguous, precloacal scales; precloacal scale row not sharply angular; multiple enlarged post-precloacal scales; two or three cloacal spurs in males; caudal scales arranged in poorly defined segments; no enlarged, plate-like subcaudal scales; band on nape; 6–8 dorsal bands lacking paravertebral elements, zig-zag to regular in shape, wider than the immaculate interspaces, not bearing lightened centers, variably edged posteriorly with light-colored tubercles, and posterior borders bold and anterior borders diffuse; usually clusters of enlarged, light-colored scales in ventrolateral fold; 11–15 immaculate light caudal bands not encircling tail; 11–15 dark caudal bands wider than light caudal bands; variably raised, moderately keeled body tubercles; and a maximum SVL of 83.8 mm. These characters are scored across all species in the gansi group in Table 3.
Re-description based on seven adults (LSUHC 13126–27, 13934–38) from the type locality. Adult SVL 69.7–83.8 mm; head moderate in length (HL/SVL 0.27–0.30), width (HW/HL 0.65–0.73), somewhat flattened (HD/HL 0.38–0.47), distinct from neck, triangular in dorsal profile; lores inflated; prefrontal region concave; rostrum concave laterally; canthus rostralis rounded; snout elongate (ES/HL 0.40–0.43), rounded in dorsal profile; eye large (ED/HL 0.23–0.27); ear opening oval to triangular in shape, moderate in size (EL/HL 0.07–0.13); eye to ear distance greater than diameter of eye; rostral rectangular, depressed medially, divided dorsally, bordered posteriorly by large left and right supranasals variably separated by small internasal, laterally by external nares and first supralabials; external nares bordered anteriorly by rostral, dorsally by one large anterior and one smaller posterior supranasal, posteriorly by 4–6 small postnasals, ventrally by first supralabial; 8–10 rectangular supralabials extending to below midpoint of eye; 9–11 infralabials tapering smoothly to below posterior margin of orbit; scales of rostrum and lores slightly raised, slightly larger than granular scales on top of head and occiput; scales on top of head and occiput intermixed with scattered, slightly enlarged tubercles; dorsal supraciliaries raised, bluntly rounded; mental triangular, bordered laterally by first infralabials and posteriorly by large, left and right trapezoidal postmentals that contact medially for 15–40% of their length posterior to mental; one row of slightly enlarged chinshields extending posteriorly to fourth infralabial; and gular and throat scales small, granular, grading posteriorly into larger, flatter, smooth, subimbricate to imbricate, pectoral scales that grade posteriorly into larger, imbricate ventral scales.
Body relatively short (AG/SVL 0.42–0.47) with well-developed ventrolateral folds; dorsal scales small, interspersed with larger, moderate to strongly keeled, semi-regularly arranged tubercles; tubercles extend from top of head onto approximately one-half the way down tail; tubercles on occiput and nape smaller and less distinctly keeled than those on posterior portion of body; approximately 16–20 longitudinal rows of dorsal tubercles; approximately 30–34 paravertebral tubercles; 39–55 flat, imbricate, ventral scales larger than dorsal scales; 11–13 contiguous, pore-bearing precloacal scales that are not sharply angled; 2–6 large post-precloacal scales with a greatly enlarged medial scale; precloacal groove or depression absent; and three cloacal spurs on each side in males.
Forelimbs moderate in stature, relatively short (FL/SVL 0.16–0.18); raised scales of forearm same size as those on body, interspersed with large tubercles; palmar scales slightly raised; digits well-developed, inflected at basal, interphalangeal joints, slightly narrower distal to inflections; claws well-developed, sheathed by a dorsal and ventral scale; enlarged series of scales at base of first digit; hind limbs more robust than forelimbs, moderate in length (TBL/SVL 0.18–0.21), covered dorsally by granular scales interspersed with large keeled tubercles and anteriorly by flat, imbricate scales; ventral scales of thigh flat, imbricate, slightly larger than dorsals, lacking a row of enlarged or pore-bearing scales; small, raised postfemoral scales grade smoothly into larger, flatter ventral scales of posteroventral margin of thigh; subtibial scales large, flat, imbricate; plantar scales raised; digits well-developed, inflected at interphalangeal joints; seven or eight expanded subdigital lamellae on fourth toe proximal to inflection, 12–15 more narrow subdigital lamellae distal to inflection, 19–23 total subdigital lamellae; and claws welldeveloped, base of claw sheathed by a dorsal and ventral scale.
Original tail moderate in proportions, 123–126% of SVL, 5.4–7.8 mm in width at base, tapering to a point; dorsal scales of base of tail small, raised but rapidly transform into larger, flatter scales posteriorly; caudal scales arranged in poorly defined segments delimited posteriorly by slightly enlarged scales; two longitudinal rows of enlarged, median, subcaudal scales not extending onto lateral margin of tail.
Coloration in life ( Fig. 5 View FIGURE 5 ). Dorsal ground color of head light-brown and body, limbs, and tail brown to paleyellow; top of head nearly unicolor, bearing weak, dark speckling; ventral portion of lores and supralabials darkened, continuing as a bold, dark, postorbital stripe forming a smooth to jagged nuchal loop extending above ear opening from posterior margin of one orbit to the other; dark loreal region, postorbital stripe, and nuchal loop highlighted dorsally by pale yellow stripe; regular to irregularly shaped, dark band on nape; nape and seven or eight regular to irregularly shaped dorsal body bands bear bold posterior and diffuse anterior borders; one postsacral band; bands not bordered posteriorly by white tubercles except for LSUHC 13937; no distinct dark markings in dorsal interspaces except in LSUHC 13934; dorsal bands wider than interspaces; clusters of enlarged, white scales in ventrolateral folds; 11–14 light caudal bands not encircling original tail; 11–14 dark caudal bands on original tail wider than light caudal bands; forelimbs dark, faintly mottled; hind limbs bearing faint, irregular banding; all ventral surfaces generally beige except for posterior portion of tail slightly darker.
Variation ( Fig. 5 View FIGURE 5 ). Body tuberculation varies from low to moderately keeled tubercles (LSUHC 13126–27, 13934–35, 13938) to more raised and strongly keeled tubercles (LSUHC 13936–37). Variation in dorsal pattern is most evident in dorsal banding ranging from broken, zig-zag, irregularly shaped dorsal bands in LSUHC 13127 and 13937 to bold, more regularly shaped bands such as those in LSUHC 13934 and 13936. The light caudal bands in LSUHC 13934–35, 13938 bear distinct dark markings not found in the other specimens. Meristic variation is presented in Table 6.
Distribution. Cyrtodactylus chrysopylos is known form the type locality of the Paydalin Cave in the Panlaung- Pyadalin Cave Wildlife Sanctuary, Ywangan Township, [Taunggyi District], Shan State, Myanmar (21.13275°N, 96.34026°E) at 303 m in elevation and the crest of the Nwalabo Mountains (21.02783°N, 96.41283°E) at 951 m in elevation, 5.1 km south of the type locality and immediately east of Yane Village ( Fig. 7 View FIGURE 7 ).
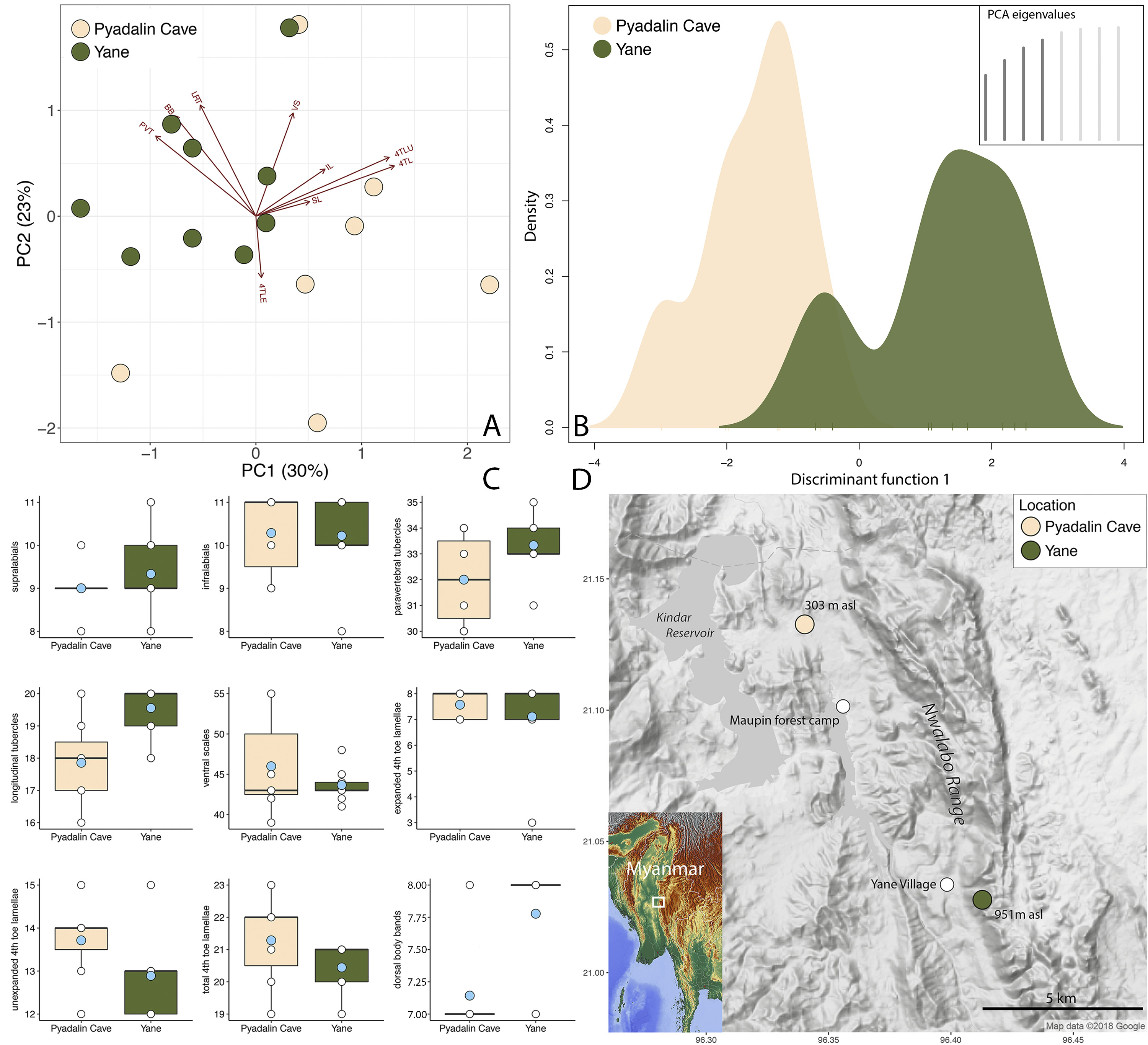
Geographic variation. Even though the PCA appears to demonstrate reasonable separation in morphospace between the Pyadalin Cave and Yane populations along PC1 and PC2 ( Fig. 7 View FIGURE 7 ), these components only account for 53% of the variation in the data set ( Table 7) and their centroids do not differ significantly (Welch two sample ttest, p = 0.47). 4TLU and 4TL account for the majority of the loadings along PC1 but ANOVA analyses did not recover their means as being significantly different. Likewise, for PVT, LRT, and BB along PC2. This indicates that the suite of characters used in this analysis do not significantly discriminate one population from another nor does any character stand out as a statistically significant contributor to interpopulational differences ( Tables 6 and 9). Four PC eigenvalues and the first discriminate function were retained for the DAPC which accounted for 81.8% of the variation and demonstrated significant overlap in the density plots for both populations ( Fig. 7 View FIGURE 7 )—also an indication they are not well-differentiated. We also found no discrete color pattern differences between the two populations although we could not confirm either the presence of absence of the bright-orange hatchling coloration of the Yane population in the Pyadalin Cave population ( Fig. 6 View FIGURE 6 ). Thus, the morphological evidence aligns itself with the genetic evidence which shows no significant interpopulational differences. Nonetheless, the Yane population tends to have more paravertebral and longitudinal rows of tubercles; more unexpanded and total subdigital lamellae on the fourth toe ( Fig. 7 View FIGURE 7 ); fewer pore-bearing precloacal scales (8–11 versus 11–13); and adults tend to be smaller (64.9–70.5 mm SVL versus 69.7–83.8 mm SVL) ( Tables 6 and 8).
Natural history. Pyadalin Cave is a limestone formation situated among the low-lying, highly eroded foothills and scree of the Nwalabo Mountains to the east lying 4.2 km north of the Maupin forest camp ( Fig. 7 View FIGURE 7 ). It consists of nine large, open chambers connected by narrow passages and three sink holes that let in natural light. The cave extends for approximately 245 m into the foot of the mountain and the interior bears several formations of flowstone, columns, stalagmites, and stalactites ( Fig. 8 View FIGURE 8 ). These and numerous bays, alcoves, tunnels, and cracks in the cave walls provide ideal microhabitat for Cyrtodactylus chrysopylos . Cyrtodactylus chrysopylos is abundant inside the cave and easily observed both day and night. During the day on 24 March 2017, we observed five specimens. Lizards were seen on stalactites up to 3 m above the cave floor, in horizontal cracks no more the 0.5 m above the cave floor, and in vertical cracks on columns 2 m above the cave floor. All were wary and quickly retreated into deeper cracks upon our approach. During the evening of 24 March 2018, we found three specimens out in the open on the walls at the cave entrance and two more on stalagmites in the interior. Several more specimens were observed but were not collected. We searched the karstic boulders, scree, and rubble immediately outside the cave
5 for the definition of the superscripts.
for approximately 200 m in each direction but found no geckos despite what looked like appropriate habitat. We also found no hatchlings or gravid females which in other Burmese species of Cyrtodactylus , are commonly found this time of year ( Grismer 2017a, 2018) and not others ( Grismer 2017b, c). Although this population appears to rely heavily on this cave for shelter, gene flow must exist between it and the Yane population 5.1 km to the south and 600 m higher in elevation along the rocky face of the Nwalabo Range ( Fig. 7 View FIGURE 7 ).
Yane Village occurs within the Panluang-Pyadalin Cave Wildlife Sanctuary and is situated in valley at 444 m in elevation between the foot of the Nwalabo mountain range and the eroded foothills to the west ( Fig. 7 View FIGURE 7 ). On the crest of the mountain above the village at 951 m in elevation is an isolated limestone outcropping on a west-facing cliff side composed of massive limestone boulders bearing several deep cracks and small caves ( Fig. 9 View FIGURE 9 ). The forest surrounding this outcropping is sparse and was being burned at the time of our visit. After dark, we observed several individuals of Cyrtodactylus chrysopylos of which we collected five adults and four hatchlings. The adults were observed on the karst from 1–4 m above the ground in both open areas and within cracks. One specimen was observed 1 m above the ground on a small branch on nearby vegetation. Three of the hatchlings were found on the ground crawling through the leaf litter and ashes near the karst and one was found low (0.25 m) on the karst. All were wary and when exposed to our light, would rapidly and often irretrievably escape into cracks in the boulders or occasionally into cracks between the ground and a boulder.
Hatchling coloration. The most striking feature of Cyrtodactylus chrysopylos is the bright-orange coloration of the hatchlings ( Figs. 6 View FIGURE 6 , 10 View FIGURE 10 ). This poses interesting questions pertaining to the adaptive significance of a nocturnal species being bright-orange and why this coloration changes ontogenetically. Many animals are active during low levels of low illumination but to comprehend the adaptive significance of an animals’ coloration—be it for crypsis or social displays—requires understanding its visual system and that of the species with which it interacts along with the environmental circumstances within which the animal is visualized ( Sumner & Mollon 2002).
Although all lizards have pure cone retinas, the visual system of most geckos has unique modifications adapted for a nocturnal life style making their night time vision 3 50–400 times more sensitive than that of humans ( Roth & Kelber 2004; Kelber & Lind 2010). These modifications include enlarged, rod-like cones with rod-specific opsins and cone visual pigments ( Kojima et al. 1992; Röll 2000) that are highly sensitive to relatively short (363–533 nm) ultraviolet, blue, and green wavelengths of light ( Crescitelli et al. 1977; Loew et al. 1996; Kelber & Roth 2006; Roth et al. 2009). This enables geckos to discriminate objects within this color range during levels of illumination that approximate that of dim moonlight ( Roth & Kelber 2004). Additionally, nocturnal geckos have relatively larger eyes, pupils, and shorter focal lengths than diurnal lizards, thus allowing even more light to expose the retina ( Kelber & Roth 2006; Schmitz & Higham 2018). However, longer wavelengths of light (597–622 nm) from the colors yellow, orange, and red fall outside the visual spectrum of the gecko species examined ( Crescitelli et al., 1977; Loew et al. 1996; Roth et al. 2009) and thus, we hypothesize the orange hatchling coloration in C. chrysopylos is not for intraspecific interactions.
A classic study by Pokorny et al. (2006) noted that in dimly lit natural environments, objects reflecting short to medium wavelengths of light (i.e. ultraviolet, blue, and green) appear brighter than objects reflecting longer wavelengths (i.e. yellow, orange, and red). In the ocean’s mesopelagic zone (150–1000 m), many species have taken advantage of this phenomenon where down-welling daylight creates extended viewscapes that become increasingly dimmer and bluer with depth. Species in the upper mesopelagic begin to take on orange or reddish colors that absorb the incident blue light, making them appear darker to match the darker color of the blue water below. In lower mesopelagic depths below 600 m, the levels of illumination are greatly reduced and orange and red pigmentation become more intense and the predominant body colors, rendering many species nearly black ( Warrant & Locket 2004). Based on this, we hypothesize that orange coloration in hatchling Cyrtodactylus chrysopylos is a form of crypsis during the low illumination levels of dusk and night time. Curiously, as hatchlings (SVL = 34.8–37.6 mm) grow, their coloration and pattern is completely transformed to that of an adult by at least a SVL of 64.9 mm. Commensurate with growth and a change in coloration, is a change in microhabitat preference from being a terrestrial leaf-litter inhabitant to a saxicolous karst boulder specialist.
The visual system of many mammals is characterized by a dichromatic retina that lacks cones sensitive to long wavelengths of light and thus shades of yellow, orange, and red do not register in their visual spectra as such ( Neitz et al. 1989). In dichromatic carnivores, such as canids, felids, and procyonids, orange coloration under low levels of illumination shifts and appears grey to dull-yellow ( Neitz et al. 1989). Nocturnal carnivores sympatric with Cyrtodactylus chrysopylos that are potential predators ( Francis 2008) include the canids Canis alpinus (Dhole) and C. lupus dingo (Dingo) ; the felids Pardofelis marmorata (Marbled Cat) , Prionailurus bengalensis (Leopard Cat) , and Catopuma temminckii (Asian Golden Cat); and the viverrids Arctictis binturong (Binturong) , Viverra zibetha (Large Indian Civet) , Viverricula indica (Small Indian Civet) , and Paguma larvata (Masked Palm Civet) — although color vision in viverrids remains unstudied. ImageJ (available at https://imagej.nih.gov/ij/download.html) was used to remove the yellow, orange, and red wavelengths from a color photograph of a hatchling C. chrysopylos in an attempt to recreate a nocturnal visual experience of a dichromatic carnivore and demonstrate the cryptic advantage of this hatchling coloration ( Fig. 10 View FIGURE 10 ). Bright colors in hatchlings of other species of Cyrtodactylus are not uncommon. For example, hatchlings in the 15 species of the C. pulchellus group (sec. Grismer & Norhayati 2008; Grismer et al. 2012, 2016) have contrasting wide, bright-yellow and black dorsal bands that in low levels of illumination, would contribute to disrupting the body outline and appear as a group of inconspicuous, dark markings on a darkly mottled karst, granite, or bark substrate.
| CAS |
California Academy of Sciences |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |