Epinephelus fuscomarginatus, Johnson & Wilmer, 2019
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4674.3.2 |
|
publication LSID |
lsid:zoobank.org:pub:6E3DF207-741B-4589-8DB3-4A3BFD16B11C |
|
persistent identifier |
https://treatment.plazi.org/id/AF30BA35-FF9F-FF98-13F6-FA65DA124FCC |
|
treatment provided by |
Plazi |
|
scientific name |
Epinephelus fuscomarginatus |
| status |
sp. nov. |
Epinephelus fuscomarginatus sp. nov.
New English name: Darkmargin Grouper
Figures 1 View FIGURE 1 , 2 View FIGURE 2 A–C, 3A–D, 4A; Tables 1–4 View TABLE 1 View TABLE 2 View TABLE 3 View TABLE 4 .
Holotype. QM I. 40945, 564 mm, Capricorn Channel , south of Swain Reefs, Qld, 22°52.8’S 152°40.8’E, 230 m, dropline, A. Lancaster, 7 Aug 2017. GoogleMaps
Paratypes. (n = 4) CSIRO H.8398-01, 456 mm, Capricorn Channel , south of Swain Reefs, Qld, 22°52.8’S 152°40.8’E, 220 m, dropline, S. Campbell, 11 Dec 2017 GoogleMaps ; QM I. 40942, 408 mm, same data as holotype GoogleMaps ; QM I. 40943, 502 mm, same data as holotype GoogleMaps ; QM I. 40944, 528 mm, same data as holotype GoogleMaps .
Diagnosis. A species of Epinephelus with dorsal-fin rays XI, 14; anal-fin rays III, 8; pectoral-fin rays 17; caudal-fin rounded; lateral-line scales 60–67; lateral-scale series 101–116; gill rakers 9–10 + 16–19 = 25–28; midlateral part of lower jaw with 2 rows of teeth; vomer v-shaped, each arm with 2–3 rows of villiform teeth; palatines narrow, with 2–4 rows of villiform teeth; angle of preopercle broadly rounded, with 4–9 small non-prominent serrae; body depth 3.0– 3.4 in SL; fourth dorsal-fin spine longest, 3.3–4.0 in HL; and colouration including broad dark brown margins to the soft dorsal, anal and caudal fins, no dark spots on the head, body, or fins at any known size, and in subadults two faint pale brown bars radiating from the eye to the edge of the opercle, and diffuse irregular brown wavy bars and blotches on the sides of the body.
Description. Morphometrics and meristics are presented in Tables 3–4 View TABLE 3 View TABLE 4 . Dorsal-fin rays XI, 14; anal-fin rays III, 8; pectoral-fin rays 17, upper ray unbranched, others including lowermost branched; pelvic-fin rays I, 5; principal caudal-fin rays 17; lateral-line scales 65 (60–67); lateral-scale series 102 (101–116); gill rakers on first arch 9 + 17 = 26 (9–10 + 16–19 = 25–28); vertebrae 24.
Body moderately deep, depth less than head length, proportionately greater with increasing length, 3.0 (3.1–3.4) in SL; body moderately compressed, greatest width 1.7 (1.6–1.8) in body depth; dorsal profile of head evenly convex, head length 2.5 (2.4–2.5) in SL; snout bluntly rounded, its length 3.9 (3.8–4.3) in HL; orbital diameter 5.5 (5.6–6.4) in HL; eyes directed laterally, interorbital space moderately convex, least width 6.0 (5.8–7.0) in HL; preorbital depth 9.2 (8.8–9.9) in HL; caudal-peduncle depth 3.7 (3.7–4.0) in HL; caudal-peduncle length 2.4 (2.5–2.6) in HL.
Mouth large, oblique, forming an angle of about 40° to the horizontal, lower jaw protruding beyond tip of upper jaw; upper jaw extending to vertical from just beyond posterior margin of pupil (just beyond posterior margin of pupil to just beyond posterior margin of eye), upper-jaw length 2.2 (2.2–2.4) in HL; posterior edge of maxilla straight (straight to slightly rounded), with narrowly rounded corners, maxilla width 8.4 (7.4–8.1) in HL. Pair of moderate canine teeth anteriorly in each jaw, those in upper jaw with gap 2.1 (1.5–2.0) times wider than gap in those in lower jaw. Teeth in lower jaw anteriorly in small patch 3–4 (3–5) teeth wide on either side of symphysis behind pair of more enlarged canines, followed posteriorly on each side by 2 rows of subequal teeth extending to rear of jaw; upper jaw with larger anterior tooth patch in 3–4 (3–5) rows, followed posteriorly by single row of enlarged teeth distally and about 3 rows of much smaller teeth mesially. Vomer v-shaped, with 2 (2–3) rows of villiform teeth; palatines narrow, with 3 (2–4) rows of villiform teeth. Tongue large, with broadly rounded tip. Longest gill raker at angle, slightly shorter than (subequal to shorter than) longest gill filament. Nostrils round, posterior nostril largest, 1.6 (1.4–1.6) times width of anterior nostril. Anterior nostril with membranous flap, apex of flap over posterior portion of rim.
Opercle with three flat spines; uppermost small, barely exposed from skin and scales, middle and lower spines much larger and distinctly exposed, middle spine longest, equidistant between upper and lower spines, tip extending further posteriorly than other spines. Upper edge of opercular membrane slightly convex, forming rounded point posteriorly. Preopercle broadly rounded at angle, with 8 small (4–9 small to moderate), non-prominent serrae, lower edge of preopercle smooth, hind edge with numerous minute serrae. Margins of subopercle and interopercle smooth.
Lateral line slightly arched over pectoral region, then becoming parallel to contour of back and straightening on peduncle. Scales cycloid on head, thorax, pectoral-fin base, abdomen and anterodorsally on body; ctenoid elsewhere on body. No auxiliary scales on body and no scales on maxilla. Minute scales on basal third of pectoral fins, basal outer half of two inner rays of pelvic fins, basal two-thirds of soft dorsal and anal fins and basal three-fourths of caudal fin.
Origin of dorsal fin above vertical from axil of pectoral fin (axil of pectoral fin to midway between axil of pectoral fin and upper corner of opercle); predorsal length 2.7 (2.6–2.8) in SL; membranes of spinous dorsal fin deeply incised; dorsal-fin spines progressively longer to fourth spine, then gradually decreasing in length to penultimate; first spine about half length of second, 8.4 (7.9–9.9) in HL; fourth spine longest, 3.7 (3.3–4.0) in HL; last dorsal-fin spine longer than penultimate, 5.5 (4.8–5.3) in HL; fifth (fifth to seventh) soft dorsal-fin ray longest, 3.1 (3.0–3.2) in HL. All dorsal and anal-fin rays branched, the last to base.
Origin of anal fin below base of second (first or second) soft dorsal-fin ray, preanal length 1.4 (1.4–1.5) in SL; first anal-fin spine short, 1.9 (1.9–2.2) in length of second spine; second anal-fin spine slightly shorter (usually slightly shorter) than third spine, 1.2 (1.0–1.2) in length of third spine, third anal-fin spine 4.8 (4.6–5.1) in HL; third soft anal-fin ray longest, length 2.8 (2.8–3.0) in HL. Caudal fin rounded, length 5.5 (5.1–5.4) in SL. Pectoral fins rounded, eighth (seventh or eighth) ray longest, 1.9 (1.7–2.0) in SL, rays branched except short uppermost and lowermost rays. Origin of pelvic fins below vertical from upper base of pectoral fins, prepelvic length 2.7 (2.7–2.9) in SL; pelvic-fin spine reaching slightly more than half distance to tip of first ray; fourth soft pelvic-fin ray longest, much shorter than longest pectoral-fin ray, length 2.5 (2.4–2.6) in HL; first pelvic-fin ray firmly attached by membrane to abdomen.
Colour when fresh. Based on fresh specimens, ground colour of upper head and body in holotype (fig. 2B) and larger paratypes uniformly dark brown; head below preopercles, thorax and abdomen pale pinkish brown. Faint broad darker brown diagonal bar from rear margin of eye to edge of opercle at third opercular spine. Smallest paratype (fig. 4A) with bar more distinct and with slightly narrower second bar from lower margin of eye crossing middle of preopercle to lower edge of opercle. Smallest paratype also with series of broad irregular diffuse wavy brown bars and blotches on sides of body, two longitudinally on upper body extending from upper opercle to below middle of soft dorsal fin and another two transversely between soft dorsal and anal fins. Spinous dorsal-fin membrane pale grey-brown in basal half, diffuse darker grey area medially, followed above adjacent to rear of each spine by dark yellowish brown pigmentation, portion of membrane posteroventral to darkly pigmented area pale and semitransparent (fig. 3A). Basal two-thirds of soft dorsal fin pale grey-brown, distal third with dark olive to honey-brown membrane (distal third dark brown in smallest paratype, see fig. 3B), contrasting with irregular submarginal pale semitransparent sections to individual rays. Basal half of anal fin pale grey, distal half dusky (fig. 3C). Caudal fin pale grey-brown on basal two-thirds, distal two-thirds diffusely dark olive-brown (fig. 3D), dark grey-brown in smallest paratype. Pectoral fin rays faintly brownish, membrane semitranslucent. Pelvic fins mostly pale and semitranslucent, distal half faintly dusky, leading edges whitish.
Colour in alcohol. Head and body of holotype (fig. 2A) and largest two paratypes (502–528 mm SL) dark greyish brown. Smallest paratype (408 mm SL) with head and body more pale brown and including two faint darker brown bars radiating diagonally from rear of eye to margin of opercle and a series of irregular broad diffuse brown wavy bars and blotches on the sides of the body. Second smallest paratype (456 mm SL) with bars on head faint, those on body barely discernible. Spinous dorsal fin membrane pale greyish brown below, an irregular darker grey cross band in midsection, and distal triangular portion semitranslucent. Soft dorsal, anal and caudal fins with irregular broad dusky margins. Paired fins pale yellowish brown.
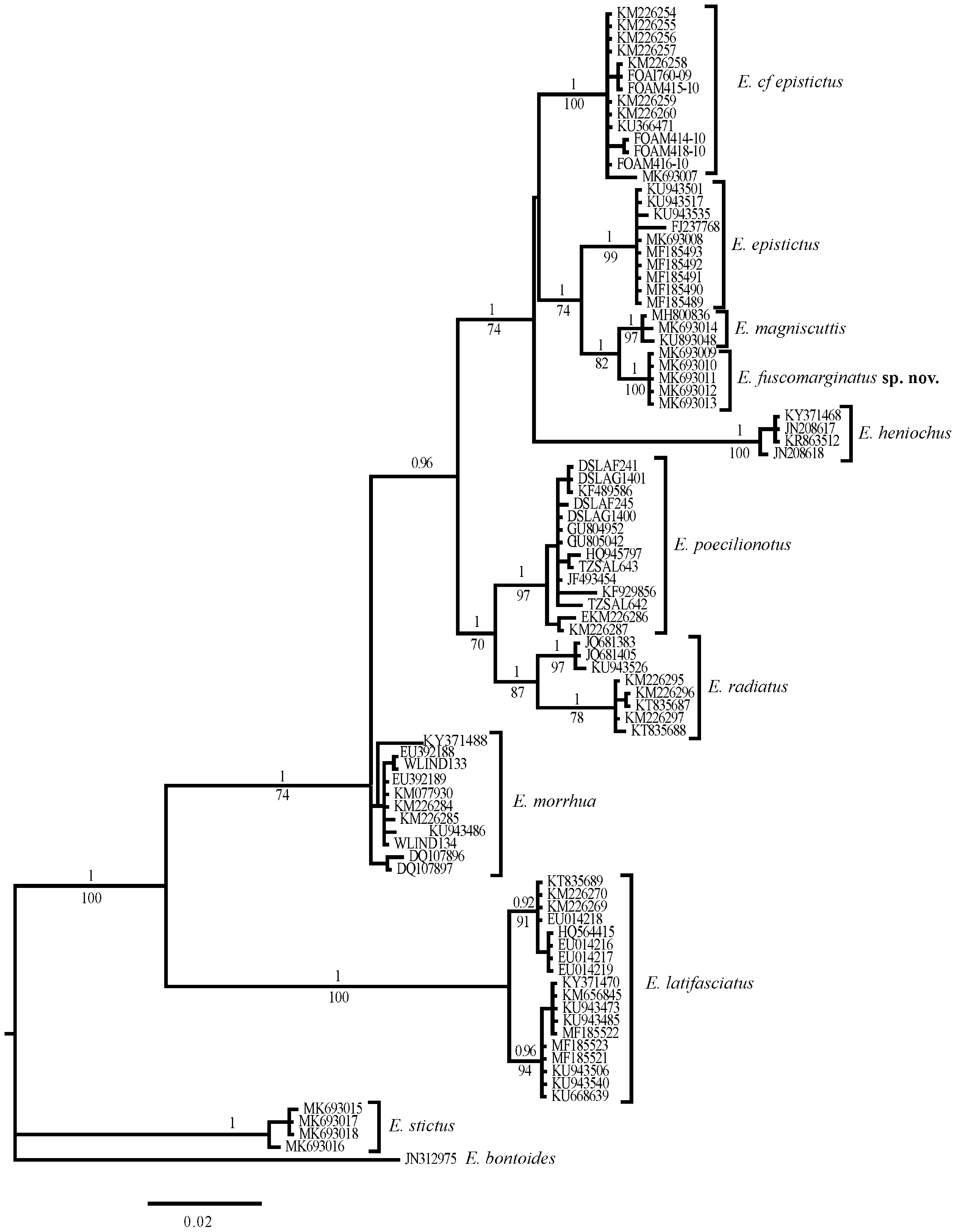
Molecular results. The phylogenetic analyses indicate that E. fuscomarginatus is most closely related to, but distinct from E. magniscuttis , E. epistictus and E. cf epistictus , with average genetic sequence divergences of 1.10%, 2.70% and 3.80%, respectively. There was no intraspecific divergence among the five samples of E. fuscomarginatus (fig. 1; Table 2 View TABLE 2 ).
Etymology. Derived from the Latin fusco for brown and marginatus for margin, in reference to the broad dark brown margins to the unpaired fins.
Distribution and abundance. The type specimens were collected by dropline from the Capricorn Channel, south of the Swain Reefs group, Qld (22°52.8’S 152°40.8’E), at depths of 220– 230 m. Epinephelus fuscomarginatus appears to have a scattered distribution within the area south of the Swain Reefs and east of the Capricorn- Bunker group. Professional fishers have reported that it can reliably be caught at a number of sites in this area, but is nowhere common. Our examination of catches of groupers from commercial deep dropline fishing off the east coast of Queensland over the past 30 years have not resulted in any additional photographs, reports, or specimens of this species outside of this area and there are no other confirmed records of the species in Australia, or elsewhere in the Indo-west Pacific.
Discussion. In the process of comparing specimens of E. epistictus to the new species, differences in colouration were noted between populations of E. epistictus from the Sea of Japan and East China Sea, versus those from the Indian Ocean east to the Indo-Australian Archipelago. As previously reported by Randall & Heemstra (1991), Heemstra & Randall (1993) and Craig et al. (2011), specimens from the northwestern Pacific usually have more numerous small dark spots on the soft dorsal and caudal fins and on the head (e.g. fig. 4B ( Japan), fig. 5A (Taiwan); Lee, 1990: fig. 51 (Taiwan); Masuda et al., 1984: pl. 117A ( Japan)), than do other populations from other regions, which often have no, or only a few spots that are mostly confined to the dorsal-fin base (e.g. fig. 4C (Arafura Sea, Australia); Randall & Heemstra, 1991: pl. 11D ( Cochin, India); Randall, 1995: pl. 297 ( Bahrain, Persian Gulf); White et al., 2013: fig. 51.28 ( Indonesia)). Higher lateral-line scale counts were also recorded for northwestern Pacific specimens (63–70 ( Randall & Heemstra, 1991) and 68–71 ( Katayama, 1960), versus 55–66 ( Randall & Heemstra, 1991) from elsewhere, which accords with the counts presented here ( Table 3 View TABLE 3 ). These morphological differences were supported by the molecular results, which indicated a genetic divergence of 3.70% ( Table 2 View TABLE 2 ) between samples available for the two populations and that each population formed a distinct, strongly supported clade (fig. 1). Consequently, the two populations are treated separately here, with those from the Indian Ocean referred to herein as E. cf epistictus . Epinephelus praeopercularis may be an available name for the Indian Ocean form, should populations from the western Indian Ocean prove to be consistent with others from throughout the broader Indian Ocean region. However, genetic samples and specimens from near the type locality (Gulf of Oman), or from elsewhere in the western Indian Ocean were unavailable, so resolution of this complex was outside of the scope of this study.
Epinephelus fuscomarginatus (fig. 2A–C; 3A–D; 4A) is most similar to E. epistictus (fig. 4B, 5A), E. cf epistictus (fig. 4C) and E. magniscuttis (fig. 4D, 6A–B; Nakamura et al., 2018, fig. 1), sharing very similar morphology and meristic formulae. It differs most obviously from all of the latter in lacking any small dark spots on the head, body, or fins at any known size. It also has irregular broad dark margins to the soft dorsal, anal and caudal fins (absent in the latter) and lacks pale or whitish outer margins to the soft dorsal and anal fins, or to the upper and lower corners of the caudal fin (usually present in E. cf epistictus ). Colouration of juveniles is unknown, but that of subadults (fig. 4A) differs markedly from E. epistictus (fig. 4B), E. cf epistictus (fig. 4C) and E. magniscuttis (fig. 4D) by the presence on the sides of the body of a series of broad irregular diffuse wavy brown bars and blotches (absent in all of the latter). Reports by commercial fishers (S. Campbell pers. comm., 2018) indicate that smaller specimens of E. fuscomarginatus of around 30–40 cm TL, which are caught and released due to minimum size restrictions, also typically have broad irregular diffuse wavy brown bars and blotches on the sides. Furthermore, in common with larger fish these smaller individuals also lack any dark spots on the head, body, or fins.
There are only minor differences in counts and proportional measurements between E. fuscomarginatus and its closest congeners. All five type specimens have 14 soft dorsal-fin rays and 17 pectoral fin rays, whereas P. epistictus , E. cf epistictus and E. magniscuttis have 14–15 (usually 15) soft dorsal-fin rays and 17–19 (usually 18) pectoral fin rays ( Table 2 View TABLE 2 ; Katayama (1960); Lee (1990); Randall & Heemstra (1991)). Lateral-line scale counts in E. fuscomarginatus are similar to E. epistictus and E. cf epistictus , but are somewhat higher than in E. magniscuttis (60–67, versus 55–62 ( Table 2 View TABLE 2 ; Randall & Heemstra, 1991). The range of total and lower arch gill-rakers for E. fuscomarginatus is slightly higher among our material examined than in the other three species ( Table 2 View TABLE 2 ). However, values for lower rakers presented by Randall & Heemstra (1991) for E. epistictus (15–18) and E. magniscuttis (16–17) are slightly higher, hence we regard the apparent variation as unreliable given the relatively low number of specimens examined. It should also be noted that in large specimens of all the above species the lowermost rakers are typically reduced to rudiments or flattened platelets that are often closely associated with their neighbour. Accurate counts are thereby difficult and minor variations in lower gill raker counts should be treated with caution, especially when obtained from different observers. In specimens greater than 400 mm SL E. fuscomarginatus has a shallower body depth than E. magniscuttis (depth in SL 3.0–3.4, versus 2.8–3.0, Tables 3 View TABLE 3 , 4 View TABLE 4 ).
Randall & Heemstra (1991) provided ratios for longest dorsal-fin spine in HL and pelvic fin in HL in E. magniscuttis as 2.45–3.0 and 2.1–2.35, respectively. This data has also been quoted by several subsequent authors (e.g. Heemstra & Randall, 1993; Craig et al., 2011). It suggests significant differences between E. magniscuttis and E. fuscomarginatus , with longest dorsal-fin spine in HL 3.3–4.0 and pelvic fin in HL 2.4–2.6 applying in the latter. However, Randall & Heemstra’s data for E. magniscuttis is at odds with that presented here (3.3–3.5 and 2.4–2.8, respectively, Table 4 View TABLE 4 ), where no significant difference in these ratios is indicated between similar-sized individuals of the two species. Differences between our data for E. magniscuttis and that of Randall & Heemstra (1991) may be most readily explained by their significantly smaller range of specimens examined (132–418 mm SL, versus 419– 613 mm SL in this study). Minor variations in several proportional measurements between E. fuscomarginatus , E. epistictus and E. cf epistictus ( Table 3–4 View TABLE 3 View TABLE 4 ) are not regarded as reliable diagnostically, due to wide variation in lengths of specimens examined between the species. Specimens of E. epistictus and E. cf epistictus 400–600 mm SL would be required for examination to determine if there are significant differences in proportional measurements between the species, but specimens of this size are generally rare or absent in international ichthyological collections.
Among other species of Epinephelus with a relatively uniform body colouration and few distinctive markings, E. fuscomarginatus differs from E. heniochus (fig. 5B) by higher lateral-line scale and gill raker counts (60–67 and 9–10 + 16–19 = 25–28, versus 54–62 and 7–9 + 14–16 = 21–25, respectively), a much larger maximum size (at least 564 mm SL, versus 350 mm SL), the lack of any small dark spots on the head and body at any known size (small yellowish brown dots sometimes present on the head and body, but often absent in large specimens and those from northwestern Australia, in E. heniochus ) and spinous dorsal-fin membrane dark brown adjacent to distal tip of spine and semitransparent posteriorly, versus distal part of membrane pale yellowish in E. heniochus . Another grouper lacking distinctive markings, Hyporthodus perplexus , known only from a single 465 mm SL specimen ( Randall et al., 1993), has fewer soft dorsal-fin rays and lateral-line scales (13 and 52, versus 14 and 60–67, respectively), a greater number pectoral-fin rays (18, versus 17), a deeper body (depth 2.9 in HL, versus 3.0–3.4) and a proportionately wider posterior nostril (about twice diameter of anterior nostril, versus 1.3–1.7). Despite a large amount of fishing effort over many years around the region of the type locality of H. perplexus (northeast of Cape Moreton, Qld, Australia, in 128–137 m), no specimens or photographs of it or E. fuscomarginatus have been reported by the numerous commercial and recreational fishers that frequent this area, or the intervening area north to off the Capricorn-Bunker Group where is latter is known. Other deep water species, E. morrhua , E. poecilonotus and E. radiatus , share similar fin ray, gill raker, lateral-line scale and lateral tooth row counts, but are readily distinguished by the distinctive markings on the sides of their bodies. They also have fewer, more enlarged serrae on the angle of the preopercle (2–5 distinctly enlarged spines, versus 4–9 non-prominent serrae in E. fuscomarginatus ) and generally have longer heads (HL 2.15–2.5 in SL, versus 2.4–2.5 in E. fuscomarginatus ) and deeper bodies (depth 2.55–3.1 in SL, versus 3.0– 3.4 in E. fuscomarginatus ).
During examination of available specimens and photographs of E. magniscuttis , considerable intraspecific variation was noted in the size and number of spots between similar-sized individuals from differing localities. Spots in specimens from Western Australia (WAM P.26610-001, 558 mm SL, WAM P.34888-001, 577 mm SL (fig. 6A) and images of four fish 60–70 cm TL (e.g. fig. 6B) supplied by C. Buckingham from off Exmouth, WA, in 250 m), although distributed in roughly similar areas of the body and fins, were much smaller and more sparse, especially on the head, soft dorsal and caudal fins, than in a specimen from Japan, KAUM I. 73560, 613 mm SL ( Nakamura et al., 2018, fig. 1). The Western Australian specimen that was genetically sampled (WAM P.34888-001) differed only by a single base pair to the Japanese specimen across the DNA barcoding fragment. As noted, the level of sequence divergence difference between E. fuscomarginatus and E. magniscuttis is low (1.10%). The number of available E. magniscuttis sequences is very small (n = 3), including one specimen from Taiwan ( KU893048 View Materials ) taken from GenBank identified as E. epistictus , but with only another single base pair difference from other E. magniscuttis sequences ( Table 1 View TABLE 1 , fig. 1). Further sequences from E. magniscuttis may provide greater genetic resolution between the two species. Iswarya Deepti et al. (2018) include three specimens identified as E. magniscuttis in their genetic (COI) identification of Indian grouper species, however, these sequences were not publically available (either through GenBank or BOLD), so could not be incorporated in this study. Other reports of E. magniscuttis from India (e.g. Sujatha et al., 2008; Sujatha et al., 2015) illustrate specimens that are doubtfully distinct from E. cf epistictus and other authors (e.g. Basheer et al., 2017) have indicated that they are likely misidentifications of E. epistictus .
Comparative materials: Epinephelus epistictus KAUM I. 34520, 190 mm, East China Sea, trawl, May 1965; KAUM I. 97319, 376 mm, off Mi-shima Island, Hagi, Yamaguchi, Japan, 34°45.16’N 131°09.19’E, line fishing, 6 Oct 2015; KAUM I. 113180 224 mm, off Ke-Zai-Liao, Ziguan District, Kaohsiung, Taiwan, K. Koeda, H. Hata & N. Muto, 5 Mar 2018. Epinephelus cf epistictus CSIRO CA. 2266, 406 mm, Arafura Sea, NT, Australia, 9°37’S 133°06’E, trawl, 126–130 m, CSIRO, 2 Jul 1981; CSIRO H.7306-03, 189 mm, Pelabuhanratu, south coast of West Java, Indonesia, 7°02’S 106°32’E, W. White, 28 Oct 2010; CSIRO H.7306-04, 191 mm, same data as previous; CSIRO H.7306-05, 210 mm, same data as previous; CSIRO H.7407-02, 199 mm, Pelabuhanratu, south coast of West Java, Indonesia, 7°02’S 106°32’E, W. White, 10 Oct 2010; MZB PB 336, 177 mm, Pelabuhanratu, south coast of West Java, Indonesia, 7°02’S 106°32’E, W. White, 28 Oct 2010; KAUM I. 12451, 146 mm, Off Kota Kinabalu, Sabah, Malaysia, 6°00’N 116°07’E, KAUM, 15 Oct 2008; NTM S.12902–028, 134 mm, NNE of Cape Don, Arafura Sea, NT, Australia, 19°19’S 132°49’E, 143 m, R. Williams, 7 Nov 1990.
Epinephelus heniochus KAUM I.22013, 95.5 mm, off Kota Kinabalu , Sabah, Malaysia, 6°00’N 116°07’E GoogleMaps , KAUM, 11 Aug 2009 ; KAUM I. 23091, 114 mm, Gulf of Thailand , KAUM, 6 Sept 2009 ; KAUM I.23282, 86.9 mm, Gulf of Thailand , KAUM, 10 Sept 2009 ; QM I. 27488, 305 mm, North West Shelf , WA, Australia, D. Tuma, 16 Nov 1990 ; QM I. 34961, 128 mm, east of Groote Eylandt , Gulf of Carpentaria, NT, Australia, trawl , CSIRO, 9 Feb 2003 .
Epinephelus magniscuttis WAM P.26610–001, 558 mm, Timor Sea, NT, Australia, 13°53’S 129°25’E, 200 m, trawl, Cliff, 4 Feb 1979 GoogleMaps ; WAM P.30510–007, 419 mm, off Ribbon Reefs , Qld, Australia, 14°04’S 145°41’E, handline, 290 m, S. Kramer & party, Jun 1992 GoogleMaps ; WAM P.34888–001, 577 mm, SW of Augusta , WA, Australia, 34°19’S 115°10’E, dropline 300 m, A. Heslewood, 20 Mar 2018 GoogleMaps .
Epinephelus poecilonotus , WAM P.31329–001, 485 mm, off Scott Reef, WA, Australia, 14°02’S 121°46’E, 162 m, trap, S. Newman, 13 Jun 1997 GoogleMaps .
TABLE 2. Estimates of mean sequence divergence within (on diagonal) and between (below diagonal) species.
| sp. nov. | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| E. cf epistictus | E. epistictus | E. magniscuttis | E. fuscomarginatus | E. heniochus | E. morrhua | E. poecilonotus | E. radiatus | E. latifasciatus | E. stictus | E. bontoides | |
| E. cf epistictus | 0.20% | ||||||||||
| E. epistictus | 3.70% | 0.10% | |||||||||
| E. magniscuttis | 3.70% | 2.40% | 0.20% | ||||||||
| E. fuscomarginatus sp. nov. | 3.80% | 2.70% | 1.10% | 0.00% | |||||||
| E. heniochus | 7.00% | 7.30% | 6.70% | 6.50% | 0.20% | ||||||
| E. morrhua | 5.10% | 5.70% | 6.10% | 6.20% | 7.80% | 0.90% | |||||
| E. poecilonotus | 4.30% | 5.00% | 5.00% | 5.50% | 8.50% | 4.00% | 0.50% | ||||
| E. radiatus | 4.40% | 4.30% | 4.70% | 4.60% | 8.10% | 4.00% | 2.70% | 1.40% | |||
| E. latifasciatus | 12.60% | 13.40% | 12.90% 12.60% | 13.70% | 11.60% | 12.10% | 11.60% | 0.80% | |||
| E. stictus | 12.50% | 11.50% | 11.80% | 12.40% | 12.60% | 11.20% | 12.00% | 12.10% | 14.50% | 0.30% | |
| E. bontoides | 15.90% | 15.60% | 15.30% 14.60% | 15.60% | 14.20% | 14.60% | 15.40% | 15.90% | 13.30% | N/A | |
TABLE 3. Meristic and morphological values for type specimens of Epinephelus fuscomarginatus sp. nov. and comparative material examined of E. epistictus, E. cf epistictus and E. magniscuttis (measurements as percentage of standard length). * Data for largest specimen of E. magniscuttis is from Nakamura et al. (2018).
| E. fuscomarginatus | E. fuscomarginatus | E. epistictus | E. cf epistictus | E. magniscuttis | |
|---|---|---|---|---|---|
| Holotype | Paratypes | non-types | non-types | non-types | |
| QM I.40945 | 4 | 3 | 8 | 4* | |
| Standard length (mm) | 564 | 408–528 | 190–376 | 134–406 | 419–613 |
| Total length | 662 | 490–626 | 236–459 | 165–502 | 507–739 |
| Dorsal-fin rays | XI/14 | XI/14 | XI/14–15 | XI/14–15 | XI/14–15 |
| Anal-fin rays | III/8 | III/8 | III/8 | III/8 | III/8 |
| Pectoral-fin rays | 17 | 17 | 18 | 18–19 | 18 |
| Lateral-line scales | 65 | 60–67 | 69–71 | 61–67 | 58–62 |
| Lateral-scale series | 102 | 101–116 | – | – | – |
| Gill rakers | 9+17 =26 | 9–10+16–19 =25–28 | 8–9+16 =24–25 | 8–10+15–17 =23–27 | 8–9+15–16 =23–25 |
| Body depth | 33.2 | 29.8–32.6 | 31.3–34.3 | 31.8–35.1 | 33.8–35.5 |
| Body width | 19.1 | 16.9–19.7 | 15.6–18.3 | 16.2–19.4 | 16.7–22.2 |
| Head length | 39.6 | 40.8–42.1 | 40.1–43.0 | 41.9–45.4 | 41.2–42.5 |
| Snout length | 10.3 | 9.8–10.7 | 8.5–9.8 | 8.6–11.0 | 10.0–11.1 |
| Orbit diameter | 7.2 | 6.6–7.3 | 6.4–8.7 | 7.0–9.2 | 6.0–7.9 |
| Preorbital depth | 4.3 | 4.2–4.8 | 3.9–4.4 | 3.1–4.0 | 3.6–4.0 |
| Interorbital width | 6.6 | 5.9–7.3 | 5.9–7.3 | 6.7–6.8 | 6.7–7.6 |
| Maxilla width | 4.7 | 5.0–5.7 | 5.1–5.4 | 5.2–5.8 | 4.8–5.6 |
| Upper jaw length | 17.7 | 17.4–18.6 | 18.6–20.2 | 19.1–19.8 | 18.7–19.5 |
| Diameter anterior nostril | 0.6 | 0.6–0.8 | 0.7–0.9 | 0.5–0.8 | 0.5–0.9 |
| Diameter posterior nostril | 1.0 | 0.8–1.1 | 1.0–1.2 | 0.7–1.1 | 1.1–1.6 |
| Caudal peduncle depth | 10.6 | 10.2–11.2 | 10.5–11.9 | 11.2–11.7 | 10.8–11.3 |
| Caudal peduncle length | 16.6 | 15.7–17.1 | 15.4–17.4 | 15.9–16.8 | 17.1–21.0 |
| Predorsal length | 36.7 | 35.3–37.9 | 35.3–39.5 | 35.8–37.4 | 35.3–38.9 |
| Preanal length | 70.7 | 68.7–70.8 | 68.4–69.4 | 68.0–70.0 | 65.2–67.9 |
| Prepelvic length | 36.5 | 35.1–37.1 | 33.9–38.4 | 36.4–38.8 | 33.3–39.6 |
| Dorsal-fin base | 52.5 | 51.6–54.4 | 50.5–54.2 | 53.0–54.9 | 53.0–55.4 |
| First dorsal spine | 4.7 | 4.1–5.3 | 4.6–6.0 | 5.7–6.7 | 5.2–5.9 |
| Second dorsal spine | 8.7 | 8.2–9.4 | 9.9–10.9 | 10.9–12.0 | 8.5–11.1 |
| Third dorsal spine | 10.0 | 10.2–11.9 | 11.7–12.8 | 13.3–14.5 | 11.7–12.9 |
| Fourth dorsal spine | 10.7 | 10.2–12.5 | 11.7–13.4 | 12.8–14.1 | 12.0–13.0 |
| Last dorsal spine | 7.3 | 7.7–8.7 | 9.0–10.7 | 9.2–11.5 | 7.5–9.5 |
| Longest dorsal ray | 12.8 | 12.9–13.6 | 14.9–17.2 | 13.9–16.7 | 13.0–14.7 |
| Anal-fin base | 14.9 | 14.9–16.5 | 16.5–17.0 | 15.4–17.4 | 15.7–16.3 |
| First anal spine | 3.5 | 3.5–4.0 | 4.0–4.9 | 3.8–5.9 | 3.2–4.5 |
| Second anal spine | 6.8 | 7.4–8.4 | 9.2–11.1 | 8.0–11.6 | 6.5–8.8 |
| Third anal spine | 8.2 | 8.0–9.2 | 9.8–11.2 | 9.8–12.3 | 7.8–9.5 |
| Longest anal soft ray | 14.1 | 13.6–14.9 | 15.7–18.5 | 14.8–17.8 | 13.6–16.1 |
| Caudal-fin length | 18.1 | 18.4–19.8 | 23.1–24.2 | 21.9–24.0 | 19.2–22.1 |
| Pectoral-fin length | 21.2 | 21.1–24.2 | 22.3–25.5 | 22.3–24.9 | 20.3–23.9 |
| Pelvic-fin length | 15.8 | 16.1–17.8 | 17.5–20.6 | 17.0–19.9 | 15.1–17.9 |
| Pelvic spine length | 8.0 | 8.8–9.2 | 10.3–11.4 | 10.3–11.8 | 8.4–10.2 |
TABLE 1. Details of all sequences analysed in this study, including Genbank/BOLD accession numbers.
| Species | Genbank/BOLD | Country |
|---|---|---|
| Epinephelus bontoides * | JN312975 View Materials /FOAM409-10 | Indonesia: West Java |
| *Species ID in Genbank: E. magniscuttis | ||
| Epinephelus cf epistictus | HM422400 View Materials /FOAMI760-09 | Indonesia: West Java |
| Epinephelus cf epistictus | JN312980 View Materials /FOAM414-10 | Indonesia: West Java |
| Epinephelus cf epistictus | JN312981 View Materials /FOAM415-10 | Indonesia: West Java |
| Epinephelus cf epistictus Epinephelus cf epistictus | JN312982 View Materials /FOAM416-10 JN312984 View Materials /FOAM418-10 | Indonesia: West Java Indonesia: West Java |
| Epinephelus cf epistictus | KM226254 View Materials (GenBank) | India |
| Epinephelus cf epistictus Epinephelus cf epistictus | KM226255 View Materials (GenBank) KM226256 View Materials (GenBank) | India India |
| Epinephelus cf epistictus | KM226257 View Materials (GenBank) | India |
| Epinephelus cf epistictus Epinephelus cf epistictus | KM226258 View Materials (GenBank) KM226259 View Materials (GenBank) | India India |
| Epinephelus cf epistictus | KM226260 View Materials (GenBank) | India |
| Epinephelus cf epistictus Epinephelus cf epistictus | KU366471 View Materials (GenBank) MK693007 View Materials (GenBank) | India Philippines: off Iloilo, Panay Island |
| Epinephelus epistictus | KU893048 View Materials (GenBank) | Taiwan |
| Epinephelus epistictus | KU943501 View Materials (GenBank) | Taiwan |
| Epinephelus epistictus | KU943517 View Materials (GenBank) | Taiwan |
| Epinephelus epistictus | KU943535 View Materials (GenBank) | Taiwan |
| Epinephelus epistictus | FJ237768 View Materials (GenBank) | Taiwan |
| Epinephelus epistictus | MF185489 View Materials (GenBank) | China: Xiamen, Fujian |
| Epinephelus epistictus | MF185490 View Materials (GenBank) | China: Haikou, Hainan |
| Epinephelus epistictus | MF185491 View Materials (GenBank) | China: Xiamen, Fujian |
| Epinephelus epistictus | MF185492 View Materials (GenBank) | China: Sanya, Hainan |
| Epinephelus epistictus | MF185493 View Materials (GenBank) | China: Sanya, Hainan |
| Epinephelus epistictus | MK639008 (GenBank) | Taiwan: Kaohsiung |
| Epinephelus fuscomarginatus sp. nov. | MK693009 View Materials (GenBank) | Australia: Capricorn Channel, south of Swain Reefs, Queensland |
| Epinephelus fuscomarginatus sp. nov. | MK693010 View Materials (GenBank) | Australia: Capricorn Channel, south of Swain Reefs, Queensland |
| Epinephelus fuscomarginatus sp. nov. | MK693011 View Materials (GenBank) | Australia: Capricorn Channel, south of Swain Reefs, Queensland |
| Epinephelus fuscomarginatus sp. nov. | MK693012 View Materials (GenBank) | Australia: Capricorn Channel, south of Swain Reefs, Queensland |
| Epinephelus fuscomarginatus sp. nov. | MK693013 View Materials (GenBank) | Australia: Capricorn Channel, south of Swain Reefs, Queensland |
| Epinephelus heniochus | KY371468 View Materials (GenBank) | China: Beibu Gulf |
| Epinephelus heniochus | JN208617 View Materials (GenBank) | N/A |
...Continued next page
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |