Geophagus pyrineusi, Deprá & Ohara & Silva, 2022
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5162.1.2 |
|
publication LSID |
lsid:zoobank.org:pub:084BF5BB-3917-49D2-A2E7-2B64C582A240 |
|
DOI |
https://doi.org/10.5281/zenodo.6798781 |
|
persistent identifier |
https://treatment.plazi.org/id/5C57D3CD-8AFA-4FA0-8FE0-6E2E93DE012B |
|
taxon LSID |
lsid:zoobank.org:act:5C57D3CD-8AFA-4FA0-8FE0-6E2E93DE012B |
|
treatment provided by |
Plazi |
|
scientific name |
Geophagus pyrineusi |
| status |
sp. nov. |
Geophagus pyrineusi , new species
( Figs. 4–8 View FIGURE 4 View FIGURE 5 View FIGURE 6 View FIGURE 7 View FIGURE 8 )
urn:lsid:zoobank.org:act:5C57D3CD-8AFA-4FA0-8FE0-6E2E93DE012B
Geophagus sp. “barra”: Ohara et al., 2017: 362, 372–373 ( Brazil, rio Teles Pires basin; diagnosis in key; short description; photo)
Holotype. MZUSP 119715 View Materials , 91.8 mm SL, Brazil, Mato Grosso State, Alta Floresta, rio Cristalino, tributary of rio Teles Pires ( rio Tapajós basin), 9°35ʹ56ʺS 55°55ʹ48ʺW, 243 m a.s.l.; W.M. Ohara & F. Talin, 8 Oct 2015. GoogleMaps
Paratypes. All from Brazil, Mato Grosso State, rio Teles Pires, rio Tapajós basin . INPA 59636 View Materials , 1 View Materials , 99.9 mm SL ; MZUSP 118122 View Materials , 1, 112.1 mm SL, same locality as holotype GoogleMaps . MZUSP 116474 View Materials , 3 View Materials , 62.6–74.3 mm SL (1 c&s, 73.2 mm SL), Paranaíta / Alta Floresta, rio Santa Helena, close to mouth at rio Teles Pires , 9°32ʹ57ʺS; 56°18ʹ55ʺW, 228 m a.s.l.; W.M. Ohara, 6 Jul 2014 GoogleMaps . MZUSP 119716 View Materials , 1 View Materials , 94.2 mm SL, Paranaíta / Alta Floresta, rio Teles Pires near mouth of rio Santa Helena, 9°33ʹ28ʺS 56°18ʹ30ʺW, 225 m a.s.l., F. Talin, 9 Oct 2015 GoogleMaps . MZUSP 119714 View Materials , 12 View Materials , 17.0– 33.7 mm SL, Alta Floresta, rio Cristalino, tributary of rio Teles Pires , 9°35ʹ56.42ʺS 55°55ʹ48ʺW, 243 m a.s.l., F. Talin, 8 Oct 2016 GoogleMaps . MZUSP 119717 View Materials , 1 View Materials , 82.1 mm SL, Alta Floresta, rio Teles Pires near São José ferry boat, 9°38ʹ22ʺS 56°00ʹ57ʺW, 233 m a.s.l., W.M. Ohara & D GoogleMaps . T.B. Nielsen , 12 Apr 2016 . MCP 30369, 6 View Materials , 37.6–42.8 mm SL, Nova Guarita / Carlinda, rio Teles Pires , 10°07ʹ07ʺS 55°34ʹ08ʺW, 238 m a.s.l. GoogleMaps , R.E. Reis, L . R. Malabarba, E.H.L. Pereira, 23 Jan 2002 . MCP 30370, 1 View Materials , 60 mm SL, Carlinda / Nova Canaã do Norte, rio Teles Pires , 10°14ʹ16ʺ S 55°48ʹ27ʺ W, 241 m a.s.l. GoogleMaps , R.E. Reis, L . R. Malabarba, E.H.L. Pereira, 22 Jan 2002 . MZUSP 116732 View Materials , 1 View Materials , 30.3 mm SL, Apiacás, rio Teles Pires , 8°22ʹ48ʺS 57°40ʹ6ʺW, 109 m a.s.l., W.M. Ohara, 14 Jan 2015 GoogleMaps . MZUSP 121509 View Materials , 1, 110.1 mm SL, Alta Floresta, rio Cristalino , 9°35ʹ9ʺS 55°55ʹ42ʺW, 242 m a.s.l., F. Talin & W.M. Ohara, 20 Aug 2015 GoogleMaps . LIT 2748, 1, not measured, Sinop, rio Teles Pires , 11°36ʹ26ʺS 55°41ʹ15ʺ W GoogleMaps , T. Colaço, J.O. Thamires, 16 Aug 2018 . LIT 1052, 2, 69.1–93.2 mm SL, Sinop, Rio Teles Pires 11°26ʹ31ʺS 55°32ʹ51ʺ W, P. Silva, J. Pereira, F. Cabeceira, G. Wolf, 15 Sep 2016 GoogleMaps . MNRJ 1277 View Materials , 1 View Materials , 80.9 mm SL, “rio Paranatinga” (currently Rio Teles Pires ) (no precise locality); A. Pyrineus de Souza, 21 May 1915 .
Diagnosis. Geophagus pyrineusi is promptly distinguished from all congeners by presenting the two anteriormost flank bars (FB5 and FB6–7) almost as dark as the midlateral spot, and as dark as the conspicuous, complete infraorbital bar (other FBs are much less intense) (vs. flank bars much less dark than the midlateral spot and the infraorbital bar, when present). In left lateral view, those three marks seem to form an N shape, which is absent in all other species. The retention throughout ontogeny of the DMP6 as a distinct mark, which becomes almost as dark as the midlateral spot in adults, and not connected to any LMPs, distinguishes G. pyrineusi from all other congeners ( Figs. 4–8 View FIGURE 4 View FIGURE 5 View FIGURE 6 View FIGURE 7 View FIGURE 8 ; vs. DMP6 completely merged into one of the anteriormost FBs; in G. megasema Heckel and G. sveni Lucinda, Lucena & Assis , the DMP6 is occasionally isolated, but never much darker than the FBs; in adult G. mirabilis , the DMPs develop into dark, isolated melanophoric patches, but they are formed by disrupted clusters of melanophores, and the DMP6 appears to be fused with the DMP5). The retention of the LMP1p in adults (vs. LMP1p developing into caudal-fin color patterns of spots, bands or stripes with growth) distinguishes G. pyrineusi from all other congeners, as well as the almost complete absence of fin patterns on dorsal and caudal fins (vs. caudal fin always with a discrete pattern of spots, vermiculations, bands or stripes in all other species, and dorsal fin usually striped, banded or spotted to some degree, even in preserved specimens). Finally, the complete absence of interradial dorsal-fin scales distinguishes G. pyrineusi from all congeners, except G. gottwaldi Schindler & Staeck and G. grammepareius Kullander & Taphorn.
Geophagus pyrineusi is further distinguished from all congeners, except G. argyrostictus , G. gottwaldi , G. grammepareius , G. harreri and G. taeniopareius , by the presence of a complete infraorbital bar (vs. only a preopercular mark in G. brachybranchus Kullander & Nijssen 1989 , G. crocatus Hauser & López-Fernández , G. dicrozoster López-Fernández & Taphorn , G. proximus (Castelnau 1855) and G. winemilleri López-Fernández & Taphorn ; and no infraorbital bar nor preopercular mark in G. abalios López-Fernández & Taphorn , G. altifrons Heckel , G. brokopondo Kullander & Nijssen , G. camopiensis Pellegrin , G. megasema , G. mirabilis , G. neambi Lucinda, Lucena & Assis , G. parnaibae Staeck & Schindler 2006 , G. surinamensis and G. sveni ). It differs from G. abalios , G. altifrons , G. argyrostictus , G. brachybranchus , G. brokopondo , G. crocatus , G. dicrozoster , G. neambi and G. surinamensis (dubious in G. gottwaldi , G. grammepareius , G, harreri , G. parnaibae and G. taeniopareius ) by the fused FB6 and FB7 (vs. not fused). It differs from G. abalios , G. altifrons , G. argyrostictus , G. brokopondo , G. mirabilis and G. neambi by the fused FB3 and FB4 (vs. not fused). It differs from G. abalios , G. altifrons , G. argyrostictus , G. neambi (with secondarily bisected FBs), G. proximus and G. winemilleri (last two with FBs only slightly bisected) by the absence of bisected FBs. The presence (vs. absence) of a supra-opercular and of a midopercular mark distinguishes G. pyrineusi from all congeners but G. crocatus , G. dicrozoster and G. mirabilis . The presence of thin lips distinguishes G. pyrineusi from G. camopiensis and G. parnaibae , which present very thick lips. The middle-sized midlateral spot, completely covering 4–9 scales, distinguishes G. pyrineusi from G. altifrons (completely covering one scale or less), G. megasema (about 14 scales) and G. proximus (about 13 scales or more).
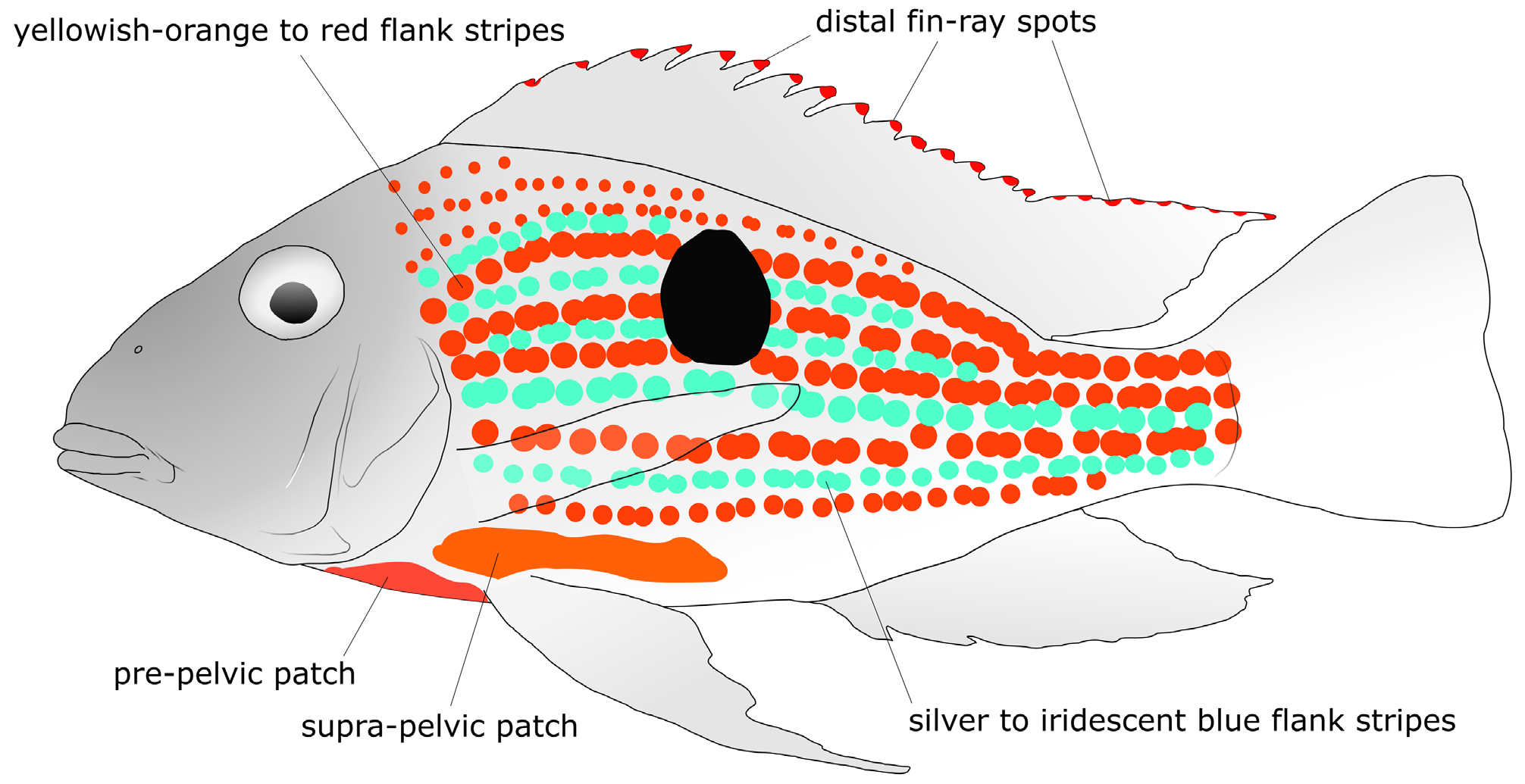
Live coloration characters useful to distinguish Geophagus pyrineusi are the absence of supra-lachrymal mark (vs. presence in G. argyrostictus , G. crocatus , G. megasema , G. mirabilis , G. neambi , and G. sveni ); absence of cheek marks (vs. presence in G. mirabilis and G. sveni ); presence of a labial mark (vs. absence in G. grammepareius , G. mirabilis , G. sveni , and G. taeniopareius ); absence of marginal opercular marks (vs. presence in G. argyrostictus , G. mirabilis and G. sveni ); absence of inner opercular marks, subopercular marks, and lachrymal marks (vs. presence in G. mirabilis ); the dark-brown eye (vs. red in G. altifrons , G. crocatus , G. dicrozoster , G. megasema , G. proximus , G. surinamensis and G. winemilleri ); the possession of distal fin-ray spots on the dorsal fin and on the dorsal margin of caudal fin (shared only with G. megasema and G. proximus ); and flank stripes ranging from orange dorsally to orangish yellow ventrally (vs. orange anteriorly to orangish yellow posteriorly in G. megasema and reddish-orange anteriorly to orange posteriorly in G. sveni ).
Description. Morphometric data in Table 1 View TABLE 1 . Maximum observed SL 113.7 mm. Head usually slightly longer than deep. Dorsal profile of head slightly convex between anterior tip of nasal bone and tip of supraoccipital. Nape profile straight, slightly ascending. Dorsal-fin origin at same vertical as anterior margin of supracleithrum. Dorsal-fin base convex, markedly descending, posteriorly ending at horizontal through middle of eye. Dorsal caudal-peduncle profile straight or slightly concave. Ventral profile of body between dentary symphysis and base of pelvic-fin spine nearly straight to slightly convex, descending. Pre-pelvic profile (along basipterygium) nearly straight, descending. Abdominal profile between pelvic-fin insertion and first anal-fin spine nearly straight, horizontal. Ventral profile of tail between base of first anal-fin spine and end of caudal peduncle nearly straight, ascending. Mouth isognathous and terminal, low on head, horizontally aligned with median pectoral-fin rays. Orbit high on head, dorsal to axial midline of flank, its center slightly posterior to midpoint of head length. Highest point of dorsal profile between bases of second and fourth dorsal-fin spines; lowest point at insertion of pelvic-fin spine. Pectoral-fin base oblique, anteriormost rays posterior to vertical through posterior margin of supracleithrum. Base of pelvic-fin spine at vertical through base of posteriormost pectoral-fin ray. First anal-fin spine insertion and last dorsal-fin spine insertion at same vertical; last anal fin soft ray inserted slightly anterior to vertical through insertion of last dorsal-fin ray. Caudal peduncle somewhat upturned, shallow, longer than deep.
Head and trunk laterally compressed. Cross-section of trunk nearly oval, broader ventrally, including caudal peduncle. Greatest width between pectoral-fin insertions, distance between pelvic-fin spine insertions little narrower. Head flat ventrally, rounded dorsally along snout. Interorbital area slightly convex. Cross-section of nape and dorsal-fin base pointed, strongly compressed; of pre-pelvic region, flat; of belly and anal-fin base, rounded; of caudal peduncle slightly rounded, almost flat dorsally.
Nostril anterior to vertical through distal tip of maxilla, slightly ventral to horizontal line through lower margin of orbit. Orbit dorsalmost point medial to orbit ventralmost point. Interorbital and internostril areas equally wide, narrower than mouth. Skin covering protruding portion of premaxillary and maxillary bones with many transverse striae. Fleshy lips of “American type ”, covering dentigerous parts of premaxillary and dentary. Upper lip fold continuous, extremely reduced medially; much broader towards lateral end of premaxilla, touching lachrymal and nasal skin when mouth closed. Lower lip evenly narrow, interrupted at dentary symphysis, not covering any of dentary lateralis foramina. Lip folds united on tip of maxilla. Thin skin flap on anterodorsal margin of orbit, along lateral border of frontal and lateral ethmoid.
E1 scales 30(5), 31*(7), 33(1); scale rows between upper lateral line and dorsal fin 5(4), 5½*(9) ventrally to base of first spine, 2(5), 2½*(7), 3(1) ventrally to base of last spine, 1(2), 1½*(4), 2(2), 2½(1) ventrally to base of last soft ray. Scale rows between those comprising lateral lines 2*(13), one specimen presenting upper lateral line descending posteriorly, and single scale series between lateral lines; scale rows between lower lateral line and first anal-fin spine, 6½ (2), 7(3) or 7½*(7). Scales in upper lateral line 20(3), 21*(6), 22(3), 23(1), and in lower lateral line 12+2(2), 12+3 (2), 13+1(1), 13+2*(4), 14+2(3), of which 7*(7), 8(2) between vertical through base of last dorsal-fin ray and distal margin of hypurals. Upper lateral line overlapping lower lateral line by 2(3), 3*(4), 5(2) scales. Anterior half of cheek naked, posterior half with mostly ctenoid scales, roughly forming 5*(6) or 6(3) series; cycloid scales on cheek distributed along anterior margin of preopercle. Scales in the infraorbital series 5(1), 6(1), 7*(5), 8(1). Opercle usually completely covered by scales, larger and ctenoid on dorsal portion, smaller and cycloid on ventral portion. Subopercle with three rows of scales, larger and ctenoid anteriorly, smaller and cycloid posteriorly, leaving variably developed narrow naked area along posteroventral margin. Anterodorsal process of subopercle and anterior margin of opercle naked. Preopercle naked, except in two specimens, one (62.6 mm SL) with two very small, cycloid scales on vertical arm on both sides, and a second one (109.7 mm SL) with three small cycloid scales on the left side only. Interopercle with few large, cycloid scales deeply embedded in skin, mostly concentrated posteriorly. Single postorbital vertical row of cycloid scales. Supracleithrum with 1(1), 2(4), 3*(2), 4(1), 6(1) irregularly sized ctenoid scales. Occipital and flank scales ctenoid. Circumpeduncular scale series 7*(13) dorsal to lower lateral line, 8*(1), 9(12) ventral to it, all ctenoid. Scales anterior to pelvic fin minute, smaller than scales between anus and pelvic fin and much smaller than flank scales, irregularly arranged. Scales between pelvic spines 5; scales between medial rays of pelvic fins 2(1), 3*(7). Predorsal scales irregularly arranged, smaller than flank scales.
Dorsal and anal fins naked, except for very few, small scales on base of last spine only on left side of one specimen (113.7 mm SL). Pectoral fin naked (6) or with 7(1) or 18(1) very small, cycloid scales only on left side, on proximal portion of lateral surface (when fin adducted). Pelvic fin scaleless, except for base of rays, covered by single scale each. Caudal fin covered mostly with tightly imbricate scales (except distal margin of fin and distal half of membranes between D2 and V2, which lack scales, and proximal half of same membranes, with only one scale series per membrane, not imbricating with scales on adjacent membranes); some inter-radial membranes with one or two scale rows.
Dorsal-fin rays XVI,10*(8), XVII,9(3), XVII,9,i(1), XVII,11(1). All dorsal- and anal-fin spines slightly curved, middle portion thickest, with groove along almost entire posterior margin. All dorsal-fin spines similarly thick; length markedly increasing from 1 st to 4 th, then slightly increasing to 7 th, equally sized from 7 th to last one. Dorsal-fin lappets short. Dorsal fin pointed at region comprised between median soft rays; no filament present in any specimens. Total pectoral-fin rays 12*(2), 13(3), 14(5), 15(3); fifth ray longest. Pelvic-fin first soft ray longest, outer branch longer than inner branch, with filamentous extension reaching middle of anal-fin base in larger specimen examined. Anal-fin rays III,7(9), III,7,i*(2), III,8(2); spines markedly thickening from first to third or second and third spines similarly thick, with only first spine much thinner; first anal-fin spine length less than half-length of 2nd; 3rd spine length less than double 2nd spine length. Middle anal-fin rays pointed, with short filamentous extension in larger specimens ( Fig. 4 View FIGURE 4 ). Caudal fin with distal margin approximately straight, slightly concave at its middle portion, without filaments; principal rays i,7,7,i*(13). Outer gill rakers on first arch 7+8(2), 7+9*(2), 8+8(1), 8+9(3), 8+10(1), 9+11(1). Width of skin fold over gill filaments (from border to closest gill raker) half-length of exposed part of outer gill filaments on middle of ceratobranchial; equal to length of exposed part of outer gill filaments on ceratobranchial extremities.
Premaxillary teeth in 3(2) or 4*(6) rows when counted near symphysis. Outer row with 15(5), 17(1), 18*(3), 19(1), 21(1) teeth, larger than those of inner rows. Teeth flexibly inserted on premaxilla, directed inwards, except those in outer row more rigidly inserted, pointing ventrally. Dentary teeth seemingly not arranged in rows in most specimens, or arranged in about four rows; more numerous near symphysis. Teeth in outer row 13(1), 14(1), 15(2), 16(1), 17*(4), 19(1), much larger than inner teeth, especially close to symphysis. One specimen with outer row of left side being replaced. Holotype with one large teeth left from previously replaced row.
Osteology. Suture between contralateral ceratobranchials 5 not including interdigitations ventrally; posteromedial teeth large, cylindrical, with large, blunt, dorsally oriented cusp and 1–2 very small, blunt, anteriorly oriented cusps; anterolaterally, teeth gradually diminishing in size; outer teeth very compressed transversally, with large, sharp, antrorse cusp and 1–2 very small, blunt, antrorse cusps. Tooth-plate length including posterolateral processes 105.8% of width; length of dentigerous area 78.3% of width; eight teeth along symphyseal margin, 22 along outer margin. Pharyngobranchial 2 with 23 teeth arranged in concentric semicircles, posteriormost ones turned anteriad. Pharyngobranchial 3 with 36 teeth arranged in 6 rows turned posteriad. Tooth plate 4 with 36 teeth arranged in 6 rows turned posteriad. Ceratobranchial 4 with 5 tooth plates, each with 3–5 teeth. One supraneural, anterior to first neural spine. Thirty-one total vertebrae, of which 14 abdominal (first 12 type A, except third, which is type A’; last two type B) and 17 caudal (first four type D’, followed by 12 type D and PU1+U1). Six posteriormost complete vertebral centra completely included in caudal peduncle. Ribs 12 abdominal and 4 caudal. Vertebrae bearing ribs, 3 rd –18 th. Caudal ribs not bifurcated; last one minute, globular, between fourth and fifth caudal vertebrae, not reaching posterior half of anal-fin base. Epineurals 11, of which first and second attached to 1 st and 2 nd vertebrae, respectively; remaining epineurals attached to ribs 3–11. Twenty-six dorsal-fin proximal pterygiophores (last one bearing last two rays; otherwise, one spine or ray for each pterygiophore), surrounded by vertebrae 1–22. Eight anal-fin proximal pterygiophores (first one bearing first two spines; last one bearing last two rays), surrounded by vertebrae 17–22 (anteriormost pterygiophore touches the anterior margin of the haemal spine of 17 th vertebra). Two epurals. One uroneural. Five branchiostegal rays. First branchial arch with 16 outer rakers (eight on epibranchial and eight on ceratobranchial) and 14 inner rakers (four on epibranchial, two between epibranchial and ceratobranchial, and eight on ceratobranchial). Second arch with 15 external rakers (four on epibranchial, one between epibranchial and ceratobranchial, nine on ceratobranchial, and one on hypobranchial) and 14 inner rakers (three on epibranchial, one between epibranchial and ceratobranchial, and ten on ceratobranchial). Third arch with 13 external rakers (two on epibranchial, one between epibranchial and ceratobranchial, and ten on ceratobranchial) and 16 inner rakers (three on epibranchial, one between epibranchial and ceratobranchial, and 12 on ceratobranchial). Fourth arch with 14 external rakers (three on epibranchial and 11 on ceratobranchial) and 18 inner rakers (two on epibranchial, one between epibranchial and ceratobranchial, and 15 on ceratobranchial). Ceratobranchial lacking rakers. Seven procurrent caudal-fin rays both dorsally and ventrally.
Color in alcohol. Background color greyish brown on anterodorsal portion of head, gradually fading to light beige or yellowish brown on posteroventral region. Intermandibular region light beige to dark brown. Branchiostegal membrane light beige to greyish brown. Infraorbital bar dark brown, extending from eye rim (ventrally to posterior margin of pupil, on second ossicle in infraorbital series posterior to lachrymal) to posterodorsal region of interopercle, through corner of preopercle. Width of infraorbital bar narrower than pupil, approximately equal along its length. Supraorbital bar dark brown, conspicuous, from posterodorsal margin of orbit to tip of supraoccipital. Roundish supra-opercular mark conspicuous in most specimens, frequently continuing dorsally as faint bar ending at horizontal line through dorsal margin of orbit. Mid-opercular mark always present, faint in some specimens. Area between pelvic and anal fins light beige. Prepelvic area (to base of pectoral fin) light beige to white.
Post-cranial marks (DMPs, LMPs and FBs) present in adults, except DMP3 (absent or fused with DMP4) in some specimens; LMP4 and FB4 (absent of fused with LMP3 and FB3, respectively; one residual bar occasionally present instead); and LMP6 and FB6 (absent of fused with LMP7 and FB7, respectively; one residual bar occasionally present instead). LMP1a and LMP1p round, greyish light brown and diffuse to very dark-brown, conspicuous. LMP1a on 0 and E1 scale series. DMP1 and FB1 frequently diffuse, latter never reaching past LMP1a ventrally. DMP2 at base of last dorsal-fin rays, typically diffuse, but conspicuous in some specimens. LMP2 at about same vertical or slightly anterior to DMP2 (coinciding with 22 nd –23 rd or 22 nd –24 th E1 scales), moderately to highly conspicuous, mostly on E1 scale series. FB2 running vertically from DMP2 to LMP2, frequently past latter to H2 scale series. DMP3 absent (possibly fused with DMP4) or present as distinct patch at middle of soft dorsal-fin base, although always less conspicuous than DMP1 and DMP2. LMP3–4 situated mostly on 17 th –18 th or 18 th –19 th E1 scales, usually forming partial longitudinal stripe with LMP2. FB3–4 usually running from DMP4 (in some specimens, partially from DMP3, forming Y shape) to H2 or H3 scale series, through LMP3–4 (FB3–4 usually more conspicuous ventrally, rather than dorsally to LMP3–4). Vestigial bar occasionally present posteriorly to FB5, possibly due to incomplete fusion of FB3 and FB4. DMP5 frequently merged into FB5 and, even when distinct, not as dark as DMP4 or DMP6. LMP5 developed into midlateral spot, completely covering about 4–9 scales distributed in E1 through E2, E3 or E4 scale series and partially covering scales up to scale series 0 ventrally and E3, E4 or E5 dorsally (precise count difficult because spot merges into FB5). Midlateral spot lying at same vertical as DMP5, or slightly anterior to it, overlapping upper lateral line somewhere between 10 th and 12 th scale. FB 5 approximately vertical, occasionally with extremities inclined posteriad, 1–2 scales wide. DMP6 almost as large as midlateral spot, 2–3 scales wide, 1½–3½ scales deep. LMP6 absent (or fused with LMP7), although residual bar present anteriorly to FB 5 in some specimens, usually connecting to LMP6 ( Figs. 4–6 View FIGURE 4 View FIGURE 5 View FIGURE 6 , 8 View FIGURE 8 ).
Color in life. See Figs. 2–3 View FIGURE 2 View FIGURE3 , 7 View FIGURE 7 . Melanophoric marks as in preserved specimens. Background color of opercle, subopercle and posterior half of cheek yellow. Infraorbital, preopercular and labial marks present, iridescent blue. Two infraorbital marks: dot immediately anterior to infraorbital bar and short stripe running from immediately posterior to infraorbital bar to halfway from preopercle. Preopercular marks united into irregularly shaped patch covering most of horizontal arm of preopercle, from right posterior to maxillary tip to anterior margin of infraorbital bar; another patch, smaller, on vertical arm, immediately dorsolateral to infraorbital bar. Labial mark covering whole upper lip, most conspicuous close to mouth corner. Flank background silver, with purplish hue dorsally. Three orange flank stripes on dorsal half of body (E1, E2 and E4 scale series). Ventral half of body with one irregular yellowishorange flank stripe along each scale series. Supra-pelvic patch present. Region along ventral arm of dentary and interopercle and prepelvic region white (pre-pelvic patch absent). Iridescent light-blue flank stripes represented by rows of spots along scales dorsal to anal-fin base and on ventral portion of caudal peduncle. Dorsal-fin background gray, each spine and soft ray with reddish orange distal fin-ray spots. Few orange stripes along last three soft rays. Light-orange blotches occupying proximal half of ventral caudal-fin lobe, somewhat continuing caudal-peduncle flank stripes. Reddish orange distal fin-ray spots on each unbranched dorsal-lobe caudal-fin ray. Pelvic and anal fins with alternating orange and iridescent light-blue stripes. Pelvic-fin stripes roughly parallel to rays, orange stripes shifting to red towards first ray and to yellow towards last ray, iridescent stripes shifting to white towards first and last rays and to light-blue on middle rays. Anal-fin stripes parallel to body axis when fin abducted, orangish stripes shifting to red towards ventral margin and to yellow towards posterodorsal margin, iridescent stripes shifting to light-blue towards ventral margin and to white towards posterodorsal margin. Pectoral-fin rays light yellow.
Ontogeny. Compare Figs. 4–5 View FIGURE 4 View FIGURE 5 , and Fig. 7A–B View FIGURE 7 . Allomery apparently absent from available sample. Moderate to strong positive allometry (R 2 usually higher than 0.5, frequently higher than 0.8) present in all characters analyzed (as percentages of SL), except caudal peduncle length (allometry absent, R 2 = 0.0176), last anal-fin spine length (subtle negative allometry, R 2 = 0.2239), pre-pectoral distance (subtle negative allometry, R 2 = 0.3626), prepelvic distance (subtle negative allometry, R 2 = 0.2689), head width (allometry absent, R 2 = 0.0647), postorbital head length (strong negative allometry, R 2 = 0.507), lower jaw length (allometry absent, R 2 = 0.1019) and orbital diameter (strong negative allometry, R 2 = 0.4731). Allochromatic features include progressive intensification of melanophoric marks, especially FB5, FB6-7, LMP1p, LMP3-4, DMP2, and DMP4. Intensity of LMP1a seems to vary regardless of SL. Regarding non-melanophoric elements, background colors of head and flank, uniformly grey in young, grows very contrasting in adults (darker on head than on flank; see description above). Allochromy in orange marks include mainly development of yellowish orange color on opercle and subopercle (absent in young specimens), and supra-pelvic patch (also absent in young specimens). In young specimens, blue iridophores on head diffuse (not forming discrete marks), mostly concentrated on infraorbital region, dorsal to upper lip, and on ventral arm of preopercle. Blue iridophores mostly concentrated between H1 and E2 scales series on flanks, forming illdefined flank stripes (in adults, those stripes located mostly dorsal to anal-fin base and on ventral portion of caudal peduncle).
Distribution. Geophagus pyrineusi is only known from the lower rio Teles Pires and its tributary, the rio Cristalino, rio Tapajós basin, Mato Grosso, Brazil ( Fig. 9 View FIGURE 9 ).
Etymology. The specific epithet pyrineusi is after Antônio Pyrineus de Sousa, who first collected the species. Alípio de Miranda-Ribeiro wrote “I dedicate this species to my good friend Lieutenant Pyrineus de Souza who rendered the Rondon Commission so good services” at the description of Hypostomus pyrineusi (Miranda-Ribeiro, 1920: 9; our translation). It was AMR’s desire to honor the collector once more, based on the specimen MNRJ 1277 ( Fig. 6B View FIGURE 6 ). By naming the species after Pyrineus de Sousa, we fulfil the original wish of this pioneer of the Brazilian ichthyology.
Habitat and ecology. Geophagus pyrineusi occurs in both clear (rio Teles Pires) and black water (rio Cristalino) rivers in moderately flowing stretches near rapids, where individuals were found over river bottoms composed by small rocks and sand. In spite of broad and intensive collecting efforts conducted at the rio Teles Pires (seven expeditions between 2014–2016), only relatively few individuals were collected, suggesting that G. pyrineusi is not an abundant species.
Conservation status. Geophagus pyrineusi was collected in a stretch of the Rio Teles Pires between the towns of Sinop and Apiacás, before the establishment of the reservoirs of the Teles Pires, São Manoel, Colíder, and Sinop hydroelectric dams. Information on the population of the species along the river after the construction of the dams are not available. However, G. pyrineusi occurs in a protected area (Parque Estadual do Cristalino), where no threats were detected.
| T |
Tavera, Department of Geology and Geophysics |
| MCP |
Pontificia Universidade Catolica do Rio Grande do Sul |
| R |
Departamento de Geologia, Universidad de Chile |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Geophagus pyrineusi
| Deprá, Gabriel De Carvalho, Ohara, Willian Massaharu & Silva, Hugmar Pains 2022 |
Geophagus sp.
| Ohara, W. M. & Lima, F. C. T. & Salvador, G. N. & Andrade, M. C. 2017: 362 |