Haplocyclops (Haplocyclops) henrii, Brancelj, Anton, 2015
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3994.4.3 |
|
publication LSID |
lsid:zoobank.org:pub:83E7EB77-EA86-4720-9679-A8204992D786 |
|
DOI |
https://doi.org/10.5281/zenodo.5615413 |
|
persistent identifier |
https://treatment.plazi.org/id/03E987EB-FFB9-C427-69DA-FA54FB2EFE2F |
|
treatment provided by |
Plazi |
|
scientific name |
Haplocyclops (Haplocyclops) henrii |
| status |
sp. nov. |
Haplocyclops (Haplocyclops) henrii sp. nov.
( Figs. 2–4 View FIGURE 2 View FIGURE 4 )
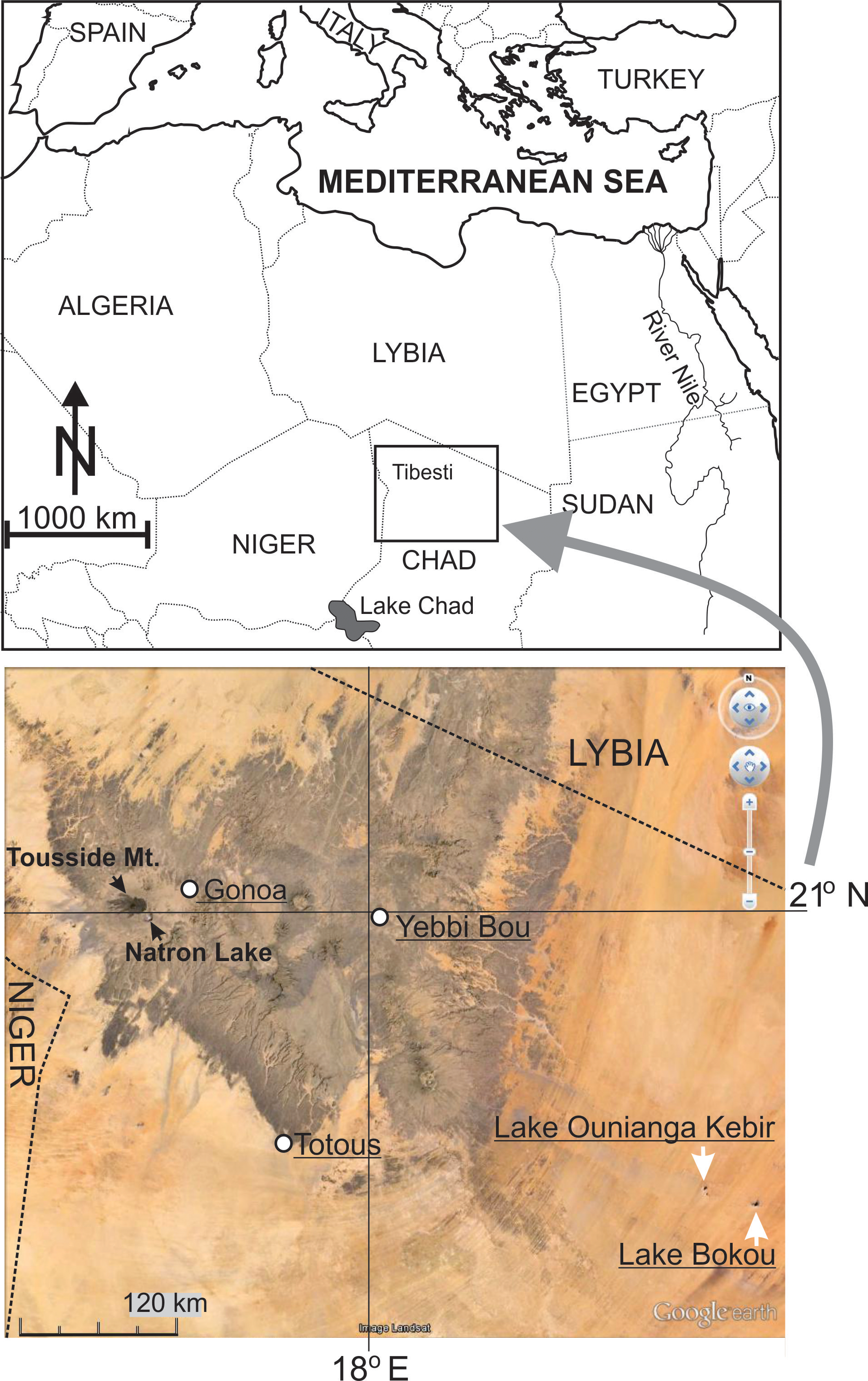
Type locality. Chad, Tibetsi area; groundwater in dry channel of river Uet Duar near village Totous, northern Chad; 19 o26'22'' N 17o31'47'' E; altitude: 585 m ( Fig. 1 View FIGURE 1 ).
Material examined. Holotype: adult female (length 357 µm), completely dissected, mounted on one slide; collected on 14 March 2014 from ground water on bottom of 1.5 m deep Karaman-Chappuis pit; access No.: PMSL-Copepoda-Brancelj-1356.
Allotype: male completely dissected, mounted on one slide; collected on the same date and same location as holotype; access No.: PMSL-Copepoda-Brancelj-1357.
Paratypes: 13 females, one female with spermatophores, 2 males; stored in 70% alcohol; collected on the same date and same location as holotype and alloype; access No.: PMSL-Copepoda-Brancelj-1358.
Additional material: 2 females, 2 male, 3 juveniles; stored in 70% alcohol in author’s collection.
FIGURE 3. Haplocyclops (Haplocyclops) henrii sp. nov., holotype female: A, P1; B, P2; C, P3; D, P4; E, P5.
Etymology. The new species is named after Prof. Henri Dumont from the University of Ghent, who is an expert for aquatic fauna of the Sahara.
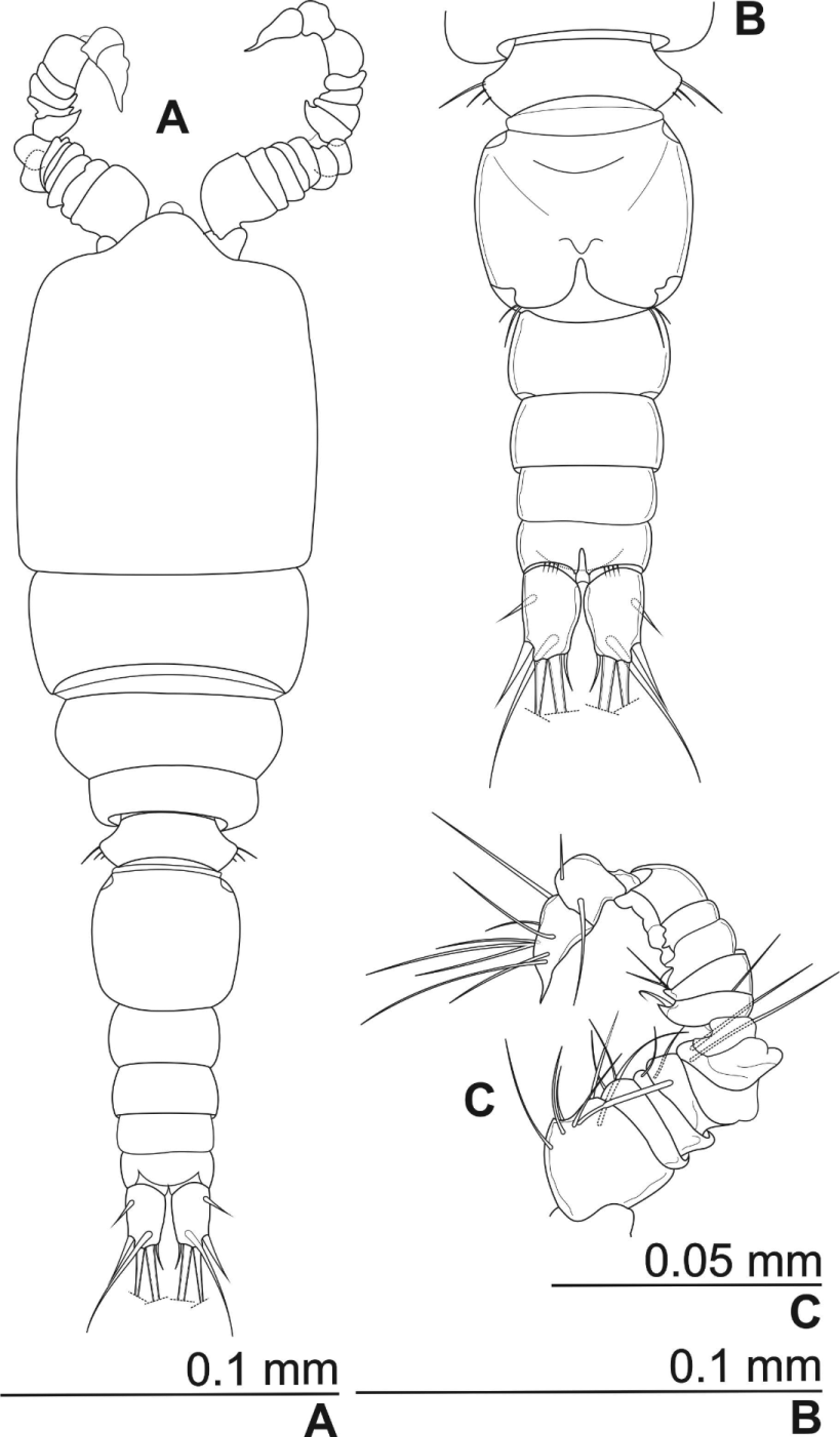
Description. Female. Body length, measured from tip of rostrum to posterior margin of caudal rami, 321–357 µm (mean body length: 339 µm; mean prosome length: 207µm; mean urosome length: 131µm; n = 8); preserved specimens colourless. Habitus elongated, almost harpacticoidal; prosomal/urosomal ratio 1.6, greatest width at anterior part of cephalothorax; cephalothorax subrectangular; compressed in dorsoventral direction ( Fig. 2 View FIGURE 2 A). Naupliar eye not discernible. Rostrum triangular with rounded tip, 2 sensilla at 2/3 of free margins length ( Fig. 2 View FIGURE 2 D).
Integumental window not discernible. Posterior margins of prosomal and urosmal somites smooth dorsally; free pedigerous somites without expansions in lateral view. Pseudo-somite between fifth pedigerous and genital doublesomite not sclerotized. Genital double-somite about 2/3 of urosome length; swollen laterally; about 1.5-times as long as wide ( Figs. 2 View FIGURE 2 A, C). Genital complex with small copulatory pore, positioned at 2/3 of genital double somite length; seminal receptacle small; anterior part expanded, posterior not discernible. Ovipores situated laterally at 2/ 3 of somite length, covered with reduced P6 ( Figs. 2 View FIGURE 2 B, C). Third and fourth urosomites without ornamentation. Anal somite with few spinules ventrally, at base of furcal rami ( Fig. 2 View FIGURE 2 C); operculum well developed, semicircular, with smooth free margin; reaching end of anal somite; with 2 sensilla at base ( Figs. 2 View FIGURE 2 A, B).
Caudal rami parallel, with some space between them ( Figs. 2 View FIGURE 2 A, C); ramus about twice as long as wide; basal part narrower than rest of ramus; no ornamentation observed. Lateral seta (I) reduced. Lateral seta (II) positioned at 1/3 of caudal ramus length, arising almost dorsally, slightly shorter than ramus length. Outermost apical seta (III) positioned subapically at 3/4 of caudal ramus length; spiniform, smooth. Outer apical seta (IV) bipinnate, about 3- times as long as caudal ramus; with no fracture plane. Inner apical seta (V) bipinnate, about 4-times as long as caudal ramus, with no fracture plane. Innermost apical seta (VI) very short, spiniform. Dorsal seta (VII) about 2- times as long as caudal ramus, smooth, uniarticulate at base; positioned close to distal end of caudal ramus on its inner corner ( Figs. 2 View FIGURE 2 A, B).
Antennule ( Fig. 2 View FIGURE 2 E) 11-segmented, not reaching middle of cephalothorax length. Segments 4 and 5 weakly chitinised, short. Aesthetascs on third and eleventh segment seta-like; both aesthetascs combined as acrotheck (common base of seta and aesthetasc; ae). Setal formula: 6.2.6+ae.1.1.1.2.2.2.2.6+ae. Lateral seta on ultimate segment present. All setae smooth.
Antenna ( Fig. 2 View FIGURE 2 F) 4 segmented, comprising long coxobasis and 3-segmented endopod. Coxobasis unornamented; one smooth seta on distal inner corner; no seta representing Exp. Short longitudinal row of spinules along inner (caudal) margin of Enp-1; one long and one short smooth seta at mid-inner margin. Enp-2 about 2- times as long as wide, armed with 3 smooth setae (one laterally, one subapically, one apically). Enp-3 2-times as long as wide, row of few spines along inner margin; 5 smooth setae apically; outermost seta on outer margin shortest and spiniform.
Mandible ( Fig. 2 View FIGURE 2 G) with 1-segmented mandibular palp; 3 smooth setae apically; 2 longest unequal in length, third very short. Ventralmost tooth on gnathobase strong, with blunt tip.
Maxillule ( Fig. 2 View FIGURE 2 H) composed of well developed praecoxa and 1-segmented palp. Arthrite of praecoxa armed with 5 armature elements along inner margin; proximal-most unipinnate; others smooth. Palp fused with Enp; 2 smooth seta laterally; 2 smooth seta apically, unequal in length.
Maxilla ( Fig. 2 View FIGURE 2 I) 5-segmented; but praecoxa partly fused with coxa. Proximal endite of praecoxa unarmed, distal with 2 smooth setae. On proximal endite of coxa one smooth seta; on distal endite 2 smooth setae, unequal in length. Basis expanded into strong, sharp-pointed claw; on concave side short row of strong small spinules; next to it robust, unipinnate seta, slightly longer than claw. Enp-1 expanded into blunt-pointed claw; one soft seta laterally, one robust, smooth seta subapically. Enp-2 very reduced; 2 smooth setae on it.
Maxilliped ( Fig. 2 View FIGURE 2 J) 4-segmented. On proximal endite of syncoxa 2 setae; one seta on distal endite. Basis 1.5- times as long as wide; with 2 smooth setae. Enp-1 short, with long unipinnate seta. Enp-2 almost as large as first one, with 3 setae unequal in length, one subapically, 2 apically.
P1–P3 with 2-segmented Exp and Enp; P4 with 2-segmented Exp, 1-segmented End (Figs. 3A–D). Armature formula of P1–P4 as in Table 2. Second exopodal segment spine formula 2.2.2.2 and setal formula 5.4.4.3.
Leg Coxa Basis Exopod Endopod
1 2 1 2
P 1 1-0 1-1 0- I 4-1 +I-I 1-0 1-1+ I-1 P 2 1-0 0-1 0- I 3-1 +I-I 1-0 1- I +1-1 P 3 1-0 0-1 0- I 3-1 +I-I 1-0 2-I +1-1 P 4 0-0 0-1 0- I 2-1 +I-I 1-1+ I-2 - P1–P4 with intercoxal sclerites; decreasing from well defined rounded distal processes at P1 and complete absence of them at P4. No spinules observed on coxae or bases. Coxae of P1–P3 with seta on inner distal margin, absent in P4. All bases on outer margin with plumose seta, decreasing in strength and length from P1 (robust, long) to P4 (soft, short). Basis of P1 with soft, short plumose seta on inner distal corner. Setae on P1–P3 End and Exp slender, plumosae; P4 End-1 with short setae (Fig. 3D). Spines on P2–P4 Exp-2 shorter than width of segment bearing them. Distal spine on P1 Exp-2 as long as width of segment bearing it.
P5 ( Figs. 2 View FIGURE 2 B, C, 3E): completely fused to somite, represented by 3 short, slender setae, unequal in length. Remnant of basal segment represented by knob-like elevation, with one long, smooth seta; 2 shorter smooth setae, representing ancestral distal segment; short setae about 0.4-times as long as longer one.
P6 ( Figs. 2 View FIGURE 2 B, C) distinct small cuticular plate with two elements; one short spiniform seta on ventrolateral side; one soft seta on dorsolateral side, about 2-times as long as short one.
Egg ( Fig. 2 View FIGURE 2 A): female with one egg attached on right side; in size similar to anterior part of genital segment.
Male. Body length, measured from tip of rostrum to posterior margin of caudal rami, 308–318 µm (mean body length: 315 µm; mean prosome length: 186 µm; mean urosome length: 129 µm; n = 3), preserved specimens colourless. Habitus elongated, almost harpacticoidal ( Fig. 4 View FIGURE 4 A); prosomal/urosomal ratio 1.4; greatest width at the cephalothorax; evenly tapering toward anal segment. Cephalothorax rectangular, width/length ratio 1.3; compressed in dorsoventral direction; rostrum similar as in female. Posterior margins of prosomites and urosomites smooth dorsally and ventrally, similar as in female ( Figs. 4 View FIGURE 4 A, B). Genital somite as wide as long. At base of furcal rami short row of spinules on ventral side of anal segment. Caudal ramus slightly conical, about 1.5-times as long as wide. Armature and position of setae similar as in female.
Antennule ( Fig. 4 View FIGURE 4 C) digeniculated; 13-segmented. Aesthetascs on segments 1 and 13. Setal formula as follows: 6+ae.2.1.1.2.0.2.2.0.0.0.2.7+ae (but some setae probably overlooked). Antenna, mouth parts (mandible, maxillule, maxilla, maxilliped) as well as P1–P5 similar as in female.
P6 ( Fig. 4 View FIGURE 4 B) partly fused medially, large cuticular plates; with 2 elements, inner seta about 3-times as long as outer one.
Spermatophore with posterior part widely rounded, anteriorly subconical; drop-like.
Variability. The only variability observed was body length.
Remarks. The genus Haplocyclops was erected by Kiefer (1952) to accommodate H. gudrunae Kiefer, 1952 from Madagascar. Later, six more species from the genus were described by Kiefer (1955, 1960), Rocha et al. (1998), Fiers (2002) and Karanovic & Ranga Reddy (2005) ( Table 3 View TABLE 3 ). Fiers (2002) also redescribed the type species and its congeners and gave a detailed discussion on relations between other related/similar genera, particularly Bryocyclops Kiefer , Rybocyclops Dussart and Palaeocyclops Monchenko. In his comparison of similarities/differences he included fine details like refractile points of the integument (present in some Bryocyclops species but absent in Haplocyclops ) and the number and size of seta of male P6 (long in Bryocyclops and reduced in Haplocyclops ). He showed that the two genera are not closely related. In contrast, Haplocyclops and Rybocyclops are probably the most closely related genera, sharing several characteristics including a large anal operculum, the dorsal position of the anteromost lateral seta on the caudal rami, the shape of the seminal receptacle and reduced armament of the legs, especially of P4. The most obvious difference between the characters of the two genera and those of Rybocyclops are: a) loss of the subdistal outer elements on the End of P1–P4, b) the absence of the medial spine on the P1, c) the spine formula 2.2.2.2 (all of those characters as apomorphies), and d) the barrelshaped female genital double-somite with the position of the gonopores in the middle of the somite sides (as a plesiomorphy) ( Fiers 2002). In his final remark Fiers stated that “the diagnosis of the genus is 2.3.3.2 (the number of spines on Exp P1–P4), but as the P4 Exp is only 1-segmented, the indication of the formula should be 2.3.3.3”. For practical reasons, however, he suggested to omit it and keep it as 2.3.3.2.
A spine formula 2.2.2.2 was suggested by Karanovic & Ranga Reddy (2005) in order to separate subgenus Haplocyclops from subgenus Kiefercyclops which had been erected to accommodate one new species from India, H. fiersi Karanovic & Ranga Reddy, 2005 , into genus Haplocyclops . Haplocyclops(Kiefercyclops) fiersi shares with other members of the genus characters like large anal operculum, principal apical seta without breaking planes, seventh antennular segment with only 2 setae, Enp of P4 1-segmented, setal formula of Exp-2 P1–P4 5.4.4.3, reduced armature of the male P6; P5 completely fused to somite and represented by three slender setae in both sexes.
There are several characters that place the new species H. (H.) dumonti sp. nov. into the genus Haplocyclops . The most obvious characters of the new species shared with the genus Haplocyclops , are, according to Kiefer (1952), a) a genital double-somite with ovipores situated in the posterior half, b) antennule without lateral seta on the ultimate segment, and c) caudal rami with lateral seta inserted in the proximal half of its length.
According to its protopodal armament of P1–P4, the new species H. (H.) henrii , with the presence of inner seta on P1–P3 and its absence on P4, fits into the subgenus Haplocyclops , not Kiefercyclops. In the subgenus Haplocyclop s, inner seta is always present on P1–P2, but either present or absent on P3 and always absent on P4. Inner seta on P3 is present in the genus in some specimens of H. (H.) monodi as well as in H. (H.) henrii . Mandibular palp, which is completely reduced in the subgenus Kiefercyclops is present in Haplocyclops , including H. (H). henrii . Kiefercyclops has 3-segmented maxilliped, which is 4-segmented in all members of the subgenus Haplocyclops . All the above mentioned characteristics of the genus are identical with a diagnosis of H (H.) henrii .
Other differential characters between H. (K.) fiersi , all six members of the subgenus Haplocyclops and the new member H. (H.) henrii , are reductions in armature and/or segmentation. According to Fiers (2002), in the subgenus Haplocyclops the setal formula of antennule is 7.2.5.2.1.2.2.2+ae.2.2+ae.6+ae while in the subgenus Kiefercyclops it is, according to Karanovic & Ranga Reddy (2005), 6.2.3.0.1.1.2.2.1.2.6, with no visible aesthetascs. Haplocyclops (H.) henrii has setal formula 6.2.6+ae.1.1.1.2.2.2.2.6+ae, which puts it in an intermediate position between the two subgenera, but closer to the subgenus Haplocyclops than Kiefercyclops. Antenna has setal formula 1.5. 7 in the subgenus Haplocyclops , 1.5. 6 in the subgenus Kiefercyclops and 1.3. 5 in H. (H.) henrii , which is the most reduced form within the two subgenera.
The most evident characters that differentiate H. (H.) henrii from the other members of the genus are: a) subrectangular shape of cephalothorax and b) very short, spiniform innermost (VI) terminal seta. There are certain other characters that are shared with some other species, but are unique in combination with other characters: c) the endopod of the fourth swimming leg, armed apically with one spine and one seta, a character shared with the South American H. (H.) torresi Rocha, Torres & Maia-Barbosa, 1998 and the Indian H. (K.) fiersi ; and d) spine formula 2.2.2.2 shared with H. (K.) fiersi , but the species differs in other characters, already listed above.
The new species fits a Gondwanaland distribution, a pattern noted in many other freshwater copepods ( Karanovic & Ranga Reddy 2005). All Haplocyclops are members of a specialized groundwater fauna which entered a subterranean environment in a distant geological past. As groundwater habitats have rather limited connectivity ( Gilbert 2001; Stoch & Galassi 2010), a specialized fauna living there has limited possibility of geographical dispersion. For that reason, a groundwater fauna is a much better indicator of ancient connections of continents than epigean fauna.
Adaptation of the new species to a groundwater environment is supported not only by a loss of pigment, absence of eye, elongated, almost vermiform body shape, reduction of appendage segments and armature, but also by reproduction. The large single egg observed in one female indicates K-selection of the species ( MacArthur & Wilson 1967), characteristic of a subterranean environment. Further specific adaptations for life in porous aquifers are weakly sclerotized and very short segments 4 and 5 on antennules, indicated also on figures in Fiers (2002) and Karanovic & Ranga Reddy (2005). Such less sclerotized parts are useful in porous aquifers where spaces between grains are small and additional flexibility of appendages enable animals to crawl among particles.
TABLE 3. Protopodal armament of P 1 – P 4 for all species of Haplocyclops, except for H. neuter (after Fiers 2002 and Karanovic & Ranga Redy 2005). The new species, H. (H.) henrii sp. nov. is included. Notation as follows: outer margininner margin coxa: outer margin-inner margin basis. Arabic numerals representing spines, Roman numerals indicating setae.
| Taxon | Distribution | Leg 1 | Leg 2 | Leg 3 | Leg 4 |
|---|---|---|---|---|---|
| Haplocyclops (Haplocyclops) pauliani Kiefer, 1955 | Madagascar | 0-1:1-I | 0-0:1-0 | 0-0:1-0 | 0-0:1-0 |
| Haplocyclops (Haplocyclops) gudrunae Kiefer,1952 | Madagascar | 0-1:1-I | 0-1:1-0 | 0-0:1-0 | 0-0:1-0 |
| Haplocyclops (Haplocyclops) monodi Kiefer, 1960 | Niger / Mali | 0-1:1-I | 0-1:1-0 | 0-0(1):1-0 | 0-0:1-0 |
| Haplocyclops (Haplocyclops) neuter Kiefer, 1955 | Madagascar | ? | ? | ? | ? |
| Haplocyclops (Haplocyclops) iranicus Fiers, 2002 | Iran | ? | 0-1:1-0 | 0-0:1-0 | 0-0:1-0 |
| Haplocyclops (Haplocyclops) torresi Rocha, Torres & Maia-Barbosa, 1998 | Brazil | 0-1:1-I | 0-1:1-0 | 0-0:1-0 | 0-0:1-0 |
| Haplocyclops (Haplocyclops) henrii sp. nov. | Chad | 0-1:1-I | 0-1:1-0 | 0-1:1-0 | 0-0:1-0 |
| Haplocyclops (Kiefercyclops) fiersi Karanovic & Ranga Reddy, 2005 | India, Andhra Pradesh | 0-0:1-0 | 0-0:1-0 | 0-0:1-0 | 0-0:1-0 |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.