Heth impalutiensis, Malysheva, Svetlana V., Mohagan, Alma B. & Spiridonov, Sergei E., 2015
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3926.1.4 |
|
publication LSID |
lsid:zoobank.org:pub:CD8D2D62-6743-4E20-BB28-647C4B3ADC90 |
|
DOI |
https://doi.org/10.5281/zenodo.5688743 |
|
persistent identifier |
https://treatment.plazi.org/id/03808789-A030-FFD9-FF1B-F895834EE290 |
|
treatment provided by |
Plazi |
|
scientific name |
Heth impalutiensis |
| status |
sp. nov. |
Heth impalutiensis n. sp.
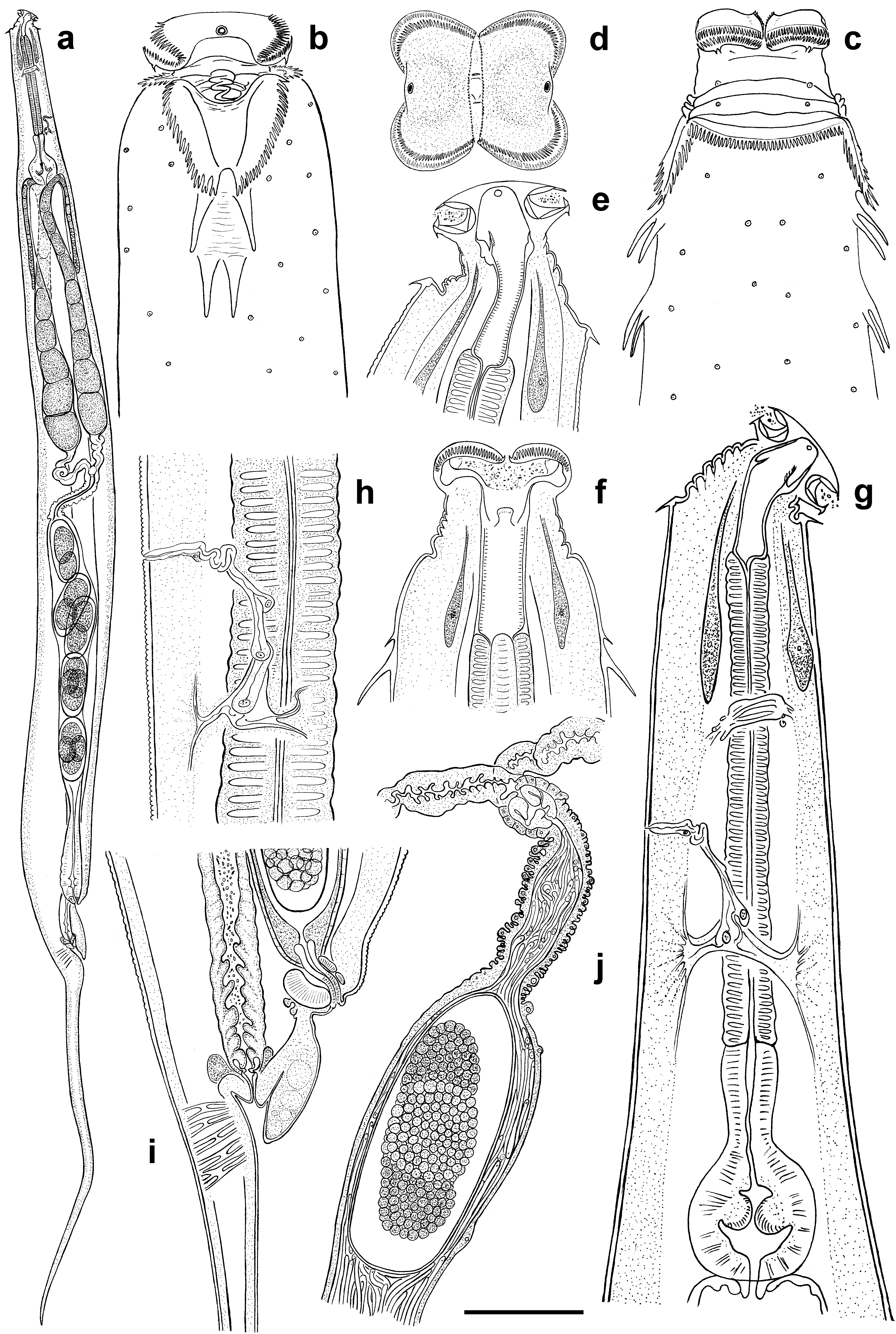
( Figs 1 View FIGURE 1 , 2 View FIGURE 2 , 3 View FIGURE 3 , 4 View FIGURE 4 )
Type host and locality. Female spirostreptid millipede ( Spirostreptida : Harpagophoridae ) collected 23 November 2013 by Mohagan A.B. and Spiridonov S.E. near Dila Falls in Impalutao barangay of Bukidnon Province, Central Mindanao, Philippines. Coordinates: 8° 14' N and 125° 02' E, 800 m above sea level (a.s.l.).
Also, representatives of described species were found 22 November 2013 in same millipede host not far from Mountain View College of Bukidnon Province. Coordinates: 7° 58' N and 125° 00' E, 680 m a.s.l.
Type material. Holotype female (No. 1250) and paratype male (No. 1251) deposited in the collection of the Centre of Parasitology, Institute of Ecology and Evolution, Russian Academy of Sciences. Paratype male (No. UGMD 104302) and paratype female (No. UGMD 104303) from the type population also deposited in the nematological collection of the Museum voor Deirkunde, Ghent University (Ghent, Belgium).
Etymology. The specific epithet is derived from Impalutao barangay (the administrative division in the Philippines) and refers to the collection site of the type specimens’ host.
Measurements. See Table 1 View TABLE 1 .
Character Dila Falls population Mountain View College population Description. Female. Cephalic extremity broad, body widening gradually behind the collar level reaching maximum diameter at mid-body then tapering posteriorly towards anus and terminating with long, subulate tail appendage ( Fig.1 View FIGURE 1 , a). Body cuticle with fine annulation 1.5 µ m apart, becoming indistinct behind vulva. Surface of each annuli bears tiny cuticular knobs ca 0.5 µ m in diam. Lateral alae absent. Head with two well developed lateral pseudolabia forming the specific shape of anterior end ( Fig.1 View FIGURE 1 , d). Free edges of each pseudolabia bear comb-like bristles reaching in length up to 5–6 µ m along dorsal and ventral sides and about 1 µ m along central slit between two pseudolabia ( Fig.3 View FIGURE 3 , c). Each subventral and subdorsal flange of cephalic plate bearing two, posteriorly directed, recurved spines. Amphidial apertures rounded, surrounded by swollen cuticular rim. Cervical collar present, comprising 48–52 posteriorly directed cuticular spines sharing a common base ( Fig. 3 View FIGURE 3 , b). Spines of unequal size increasing in length from center to the lateral edges. Collar continuous, except laterally where it extends posteriorly forming pairs of cuticular lappets with serrate outer margins ( Fig. 1 View FIGURE 1 , b, c; Fig. 3 View FIGURE 3 , a). Inner margins of lappets delimit trapezium-shaped region of smooth cuticle. Two lateral pairs of large, posteriorly directed spines on each side of body arranged in tandem posterior to lappets. Lateral spines 28–32 µ m long, divided by area of wrinkled cuticle. Bases of posterior pair are most approximated. Numerous somatic papillae present on the body cuticle.
Buccal cavity 61–63 µ m long, divided into three parts ( Fig. 1 View FIGURE 1 , e, f). Anterior part, situated between pseudolabia, is followed by middle one 14–15 µ m long provided with three cuticular plates. Plates are dorsal and subventral in position, bearing minute bristles at its inner side. Posterior part of buccal cavity ca 31–32 µ m long with distinctively striated walls. Pharynx divided into slender, cylindrical corpus, clearly delimited isthmus and rounded basal bulb ( Fig. 1 View FIGURE 1 , g). Nerve ring 146 ± 15 (125–163) µ m from anterior end. Excretory pore 235 ± 28 (185–275) µ m from anterior extremity followed by sinuous duct composed by at least four cells and leading into transverse sinus located posteriorly ( Fig. 1 View FIGURE 1 , h). Two excretory ducts extend laterally from each side of the sinus toward both ends of the body becoming undistinguishable under light microscope. Intestine long, straight, filled with food content. Vulva slightly salient, 116 ± 15 (100–150) µ m anterior to anus, body narrowing abruptly posterior to its level. Anterior anal lip strongly developed, overlapping anal aperture ( Fig. 1 View FIGURE 1 , i). Genital tract didelphic, prodelphic. Muscular vagina leads to thin-walled uteri filled with a mass of filiform spermatozoa ( Fig. 1 View FIGURE 1 , j) and containing up to 10 eggs. Eggs 177 ± 11.2 (155–190) × 82 ± 8 (70–90) µ m in size, with thin flexible shell. Tail very long, subulate.
Male. Cephalic extremity narrow, body reaching its maximum diameter at mid-body and tapering posterior to ventral sucker gradually moving into tail appendage ( Fig. 2 View FIGURE 2 , d). Body cuticle finely annulated, annules 1.2 µ m apart ( Fig. 2 View FIGURE 2 , c). Lateral alae absent. Oral opening situated apically, surrounded by thin cuticular band projecting from the lumen ( Fig. 3 View FIGURE 3 , d). Amphidial apertures rounded, surrounded by thin cuticular rim. Numerous somatic papillae present on the body cuticle. Buccal cavity ca 32 µ m long, consisting of two parts: anterior part lined by thin cuticule and thick-walled posterior part with one dorsal and two subventral cuticular formations projecting into buccal cavity ( Fig. 2 View FIGURE 2 , a, b). Each cuticular formation consists of a central stalk surrounded by cuticular lamellae resembling rose petals. Pharyngeal corpus long, fusiform with max. diam. of 33 µ m at its middle. Isthmus well defined, basal bulb rounded with strongly cuticularised valves. Nerve ring 173 ± 28 (125–212) µ m from anterior end. Excretory pore 277 ± 25.4 (250–312) µ m from anterior extremity, arranged similarly to that of female. Intestine straight, tapering posterior to basal bulb. Testis flexure 196 ± 59 (135–300) µ m posterior to basal bulb. Vas deferens subdivided into three regions: anterior with granular content; swollen middle region and posterior mostly composed of polygonal cells and connected with cloaca. Ventral sucker rounded, 30–35 µ m diam., situated 175–205 µ m anterior to cloacal aperture. Narrow channel located at the base of sucker chamber leads into spherical cavity with light, fibrous content ( Fig. 2 View FIGURE 2 , e). Spicule single, canoe-like, with two equal heads ( Fig. 2 View FIGURE 2 , i, h; Fig. 3 View FIGURE 3 , f). Spicule axis slightly curved close to distal end. Gubernaculum trowel-like, with curved lateral edges ( Fig. 2 View FIGURE 2 , j). Seven pairs of genital papillae present: one pair of mammiform papillae situated subventrally, posterior to ventral sucker; two pairs precloacal, of which posterior-most located on the sides of cloaca aperture; one pair lateral at cloaca level and three pairs postcloacal, of which two pairs situated ventrally and one pair dorsolaterally ( Fig. 2 View FIGURE 2 , f, g; Fig. 3 View FIGURE 3 , e). Cuticularised median duct is well defined on anterior cloacal lip. Low bursa-like fold of cuticle extends from behind the cloaca level to base of filiform tail appendage. Tail initially conoid, rapidly attenuating into filiform tip.
Diagnosis and relationships. Heth impalutiensis n. sp. is characterized by the presence of a cervical collar consisting of unequal sized spines, the shape of the lateral lappets and form of anterior anal lip. Males possess a low bursa-like extension around the cloacal aperture and a mammiform pair of precloacal papillae situated directly behind the ventral sucker.
Twelve Heth species have been described to date from the Australasian ( Australia, New Zealand) and Asia- Pacific region ( Papua New Guinea, Philippines, Sumatra, Vietnam). The morphology of the cervical collar, lateral lappets and cuticle ornamentation of the species described herein is mostly related to the following 11 species: Heth juli Cobb, 1898 , Heth costata Hunt, 1994 , Heth dimorphum Chitwood, 1935 , Heth xaniophora Hunt, 1994 , Heth sutherlandi Hunt, 1994 , Heth ortonwilliamsi Hunt, 1994 , Heth vietnamensis Malysheva & Spiridonov, 2010 , Heth taybaci Malysheva & Spiridonov, 2010 , Heth taynguyeni Malysheva & Spiridonov, 2010 , Heth tonkinensis Malysheva & Spiridonov, 2010 and Heth baudini Malysheva & Cribb, 2012 .
Females of H. impalutiensis n. sp. can be easily differentiated from H. costata and H. xaniophora by the absence of longitudinal cuticular ridges and lateral alae, respectively. The bases of lateral spines are widely spaced in H. impalutiensis n. sp., whereas fused in H. juli and H. tonkinensis . Heth impalutiensis n. sp. is close to H. sutherlandi and H. ortonwilliamsi in terms of the closer spaced bases of the posterior pair of lateral spines, but in H. impalutiensis n. sp. the spines are of equal size while in H. sutherlandi the posterior pair is longer but shorter in H. ortonwilliamsi . Heth impalutiensis n. sp. can be differentiated from H. baudini by bigger size of lateral spines (28–32 µ m vs 13–15 µ m, respectively) and the overall shape of the lateral lappets. Body size (L) in females of H. impalutiensis n. sp. is significantly larger than that of H. taybaci and H. taynguyeni (2610–4225 µ m (in Dila Falls population) and 3050–3450 µ m (in Mountain View College population) vs 2150, 1905 µ m and 1365–2075 µ m, respectively). Females of H. impalutiensis n. sp. are relatively similar to females of H. dimorphum and H. vietnamensis in body size and form of lateral lappets, but can be distinguished by the longer tail (750–1300 µ m vs 470–670 1 µ m and 463–510 µ m, respectively). The presence of a bursa-like fold of cuticle in males H. impalutiensis n. sp. makes them similar to the following representatives of the genus: H. xaniophora , H. ortonwilliamsi , H. taybaci , H. taynguyeni , H. baudini and two New Guinean species, described as Heth sp. ‘A’ and Heth sp. ‘B’ ( Hunt 1994). The bursa-like fold in H. impalutiensis n. sp. is formed only by the body cuticle, whereas in H. baudini it has some embedded somatic papillae. Heth impalutiensis n. sp. can be also differentiated from the latter species by the rounded form of the ventral sucker vs elliptical. In Heth sp. ‘A’, Heth sp. ‘B’ and H. taybaci the bursa-like fold extends from the anterior pair of precloacal papillae, whereas in the present species it begins behind cloaca level. Heth impalutiensis n. sp. also varies from Heth sp. ‘A’ by presence vs absence of cuticular formations inside the buccal cavity. Males of H. impalutiensis n. sp. can be easily distinguished from those of H. ortonwilliamsi and H. taynguyeni by spicule shape (canoe-like with pointed distal tip vs boat-like in H. ortonwilliamsi and distally bifurcate in H. taynguyeni ). The combination of such morphological features as presence of a bursa-like cuticular fold, the form of spicules and development of mammiform papillae make H. impalutiensis n. sp. mostly similar to H. xaniophora , which can be distinguished by having one fewer pair of genital papillae.
Heth impalutiensis n. sp. can be distinguished from all nominal species described from this geographical region by hypertrophy of the anterior anal lip which overlaps the anal aperture.
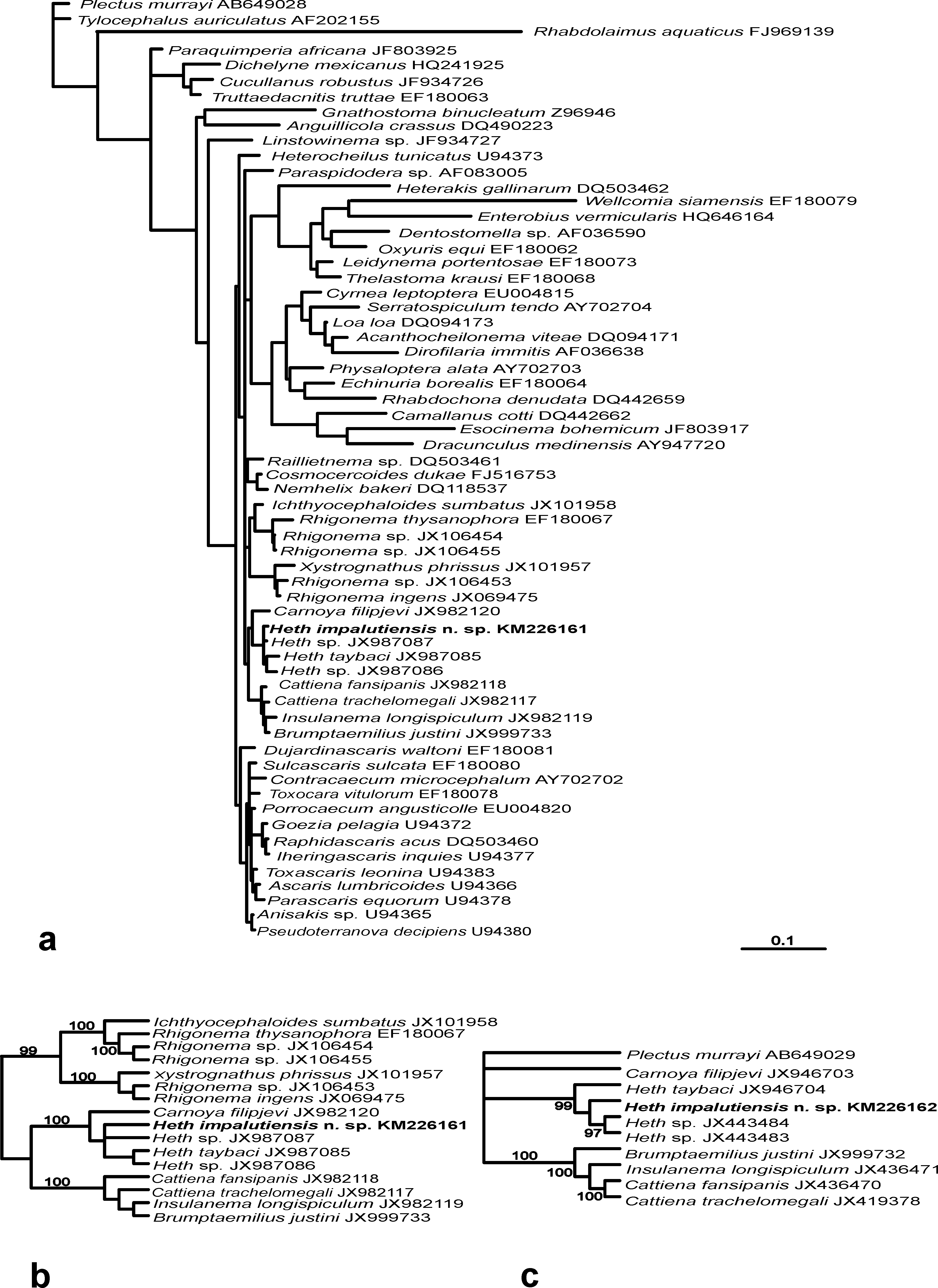
Phylogenetic relationships. The phylogenetic analysis of the 18S rDNA sequence of H. impalutiensis n. sp. was used to define the phylogenetic links of this new species ( Fig. 4 View FIGURE 4 , a). An undescribed Heth sp. ( JX987087 View Materials ) from Indonesia is the closest to the species described herein with only 4 bp nucleotide differences present. Two other representatives of Heth with known 18S rDNA data differ from described the Philippine specimens by 11 and 14 bp ( Heth sp. ( JX987086 View Materials ) from Australia and H. taybaci ( JX987085 View Materials ) from Vietnam, respectively). In the 18S rDNA tree H. impalutiensis n. sp. forms a cluster with other Heth species with 100% posterior probability ( Fig. 4 View FIGURE 4 , b). A high level of support (99%) is also observed in the cladogram for the same four Heth species obtained with Bayesian analysis of partial 28S rDNA sequence (D2-D3 expansion segment) ( Fig. 4 View FIGURE 4 , c). The only difference is in the position of H. taybaci ( JX946704 View Materials ), which is situated in a basal position in the 28S tree for the genus, with H. impalutiensis n. sp. forming a single, 99%-supported, group with the remaining two species. Heth sp. ( JX443484 View Materials ) from Australia is the closest with 19 bp difference with H. impalutiensis n. sp., while the other two species differ by 28 and 38 bp ( Heth sp. ( JX 443483 View Materials ) and H. taybaci ( JX946704 View Materials ), from Indonesia and Vietnam, respectively).
TABLE 1. Morphometrics of Heth impalutiensis n. sp. All measurements are in µm and in the form: mean ± standard deviation (range).
| Females | Males | Females | Males | |
|---|---|---|---|---|
| Holotype Paratypes | Paratypes | Vouchers | Vouchers | |
| n | – 8 | 6 | 9 | 8 |
| L | 3215 3464 ± 618 (2610–4225) | 1923 ± 255 (1595–2200) | 3182 ± 132 (3050–3450) | 1739 ± 109 (1550–1875) |
| L ′ | 2015 2494 ± 481 (1860–3115) | 1764 ± 248 (1425–2000) | 2247 ± 57 (2165–2350) | 1590 ± 111 (1375–1725) |
| a | 16 16.8 ± 3 (15.4–24) | 13 ± 1.8 (10.4–16) | 12.2 ± 2.1 (13–20) | 11.3 ± 1.3 (10.2–14.2) |
| b | 7.4 8 ± 1.3 (7–10.3) | 3.3 ± 0.5 (2.7–4) | 7.7 ± 0.4 (7.2–8.4) | 3.1 ± 0.2 (2.7–3.4) |
| c | 2.6 4 ± 0.3 (3.2–4.2) | 13 ± 2.3 (9.3–16.4) | 3.4 ± 0.2 (3–3.5) | 12 ± 1.9 (8.8–15) |
| c ′ | 13.3 13.1 ± 3.3 (8–19) | 3.3 ± 0.6 (2.8–4.3) | 13 ± 1.4 (10–15) | 2.6 ± 0.6 (1.7–3.5) |
| V | 60 68 ± 2.6 (67–74) | – | 67.1 ± 1.4 (65–69) | – |
| V ′ | 96 95.2 ± 0.9 (94–97) | – | 95 ± 0.4 (95–96) | – |
| Pharynx length | 430 442 ±36.1 (400–500) | 579 ± 25 (550–600) | 411 ± 23 (380–460) | 553 ± 48 (465–625) |
| Head to vulva | 1930 2379.3 ± 475 (1750–2965) | – | 2136 ± 54.1 (2050–2225) | – |
| Max. body diam. | 200 198 ± 40.2 (140–250) | 149 ± 31 (130–210) | 200 ± 22.4 (175–240) | 154 ± 19 (121–175) |
| Anal/cloacal body diam. | 90 80 ± 30 (40–125) | 47.2 ± 7.8 (40–60) | 75.2 ± 6.2 (70–90) | 59 ± 11 (43–75) |
| Spicule length (arc) | – – | 138.3 ± 10.7 (128–158) | – | 133 ± 6.4 (123–140) |
| Gubernaculum length | – – | 62 ± 6.2 (55–73) | – | 63 ± 7 (55–75) |
| Tail | 1200 970 ± 172.4 (750–1300) | 154 ± 19.1 (125–170) | 947 ± 84 (850–1100) | 149 ± 21 (113–175) |
| UGMD |
Zoology Museum of the University of Ghent |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Heth impalutiensis
| Malysheva, Svetlana V., Mohagan, Alma B. & Spiridonov, Sergei E. 2015 |
Heth baudini
| Malysheva & Cribb 2012 |
Heth vietnamensis
| Malysheva & Spiridonov 2010 |
Heth taybaci
| Malysheva & Spiridonov 2010 |
Heth taynguyeni
| Malysheva & Spiridonov 2010 |
Heth tonkinensis
| Malysheva & Spiridonov 2010 |
Heth costata
| Hunt 1994 |
Heth xaniophora
| Hunt 1994 |
Heth sutherlandi
| Hunt 1994 |
Heth ortonwilliamsi
| Hunt 1994 |
Heth dimorphum
| Chitwood 1935 |
Heth juli
| Cobb 1898 |