Tribodus limae Brito and Ferreira, 1989
|
publication ID |
https://doi.org/ 10.1206/681.1 |
|
persistent identifier |
https://treatment.plazi.org/id/038E7B7E-FF8B-7E3D-AF42-FD4DFD157410 |
|
treatment provided by |
Carolina |
|
scientific name |
Tribodus limae Brito and Ferreira, 1989 |
| status |
|
Tribodus limae Brito and Ferreira, 1989
TYPE SPECIES: Tribodus limae Brito and Ferreira, 1989 .
DIAGNOSIS: As for the type species, Brito and Ferreira, 1989. Hybodont shark, weakly heterodont. Teeth high crowned not exceeding 5 mm in length, crown with strong vascular striae, deeper than broad in anterior and posterolateral teeth, shallower than broad in anterolateral teeth, root hybodontoid; fin spines slender reaching about 12.5 cm, lateral faces with 8 to 10 sharp continuous ridges, single anterior ridge forming a keel in the anterior border, posterior denticles in two series; large dermal denticles with thornlike shape measuring approximately 2.5 to 3.0 mm.
An emended diagnosis for Tribodus limae was provided by Brito (1991), in agreement with that of Brito and Ferreira (1989) except for the following change:
Hybodont shark of approximately 700 mm total length; teeth weakly heterodont [heterodonty is monognathic; possibly also dignathic], higher than broad… tooth crown with striae, deeper than broad in posterolateral teeth and shallower than broad in anterior and antero-lateral teeth; root much more developed than the crown, showing in labial view a central foramen.
NEUROCRANIUM
GENERAL FEATURES: The neurocranium of
Tribodus limae was first described by Brito
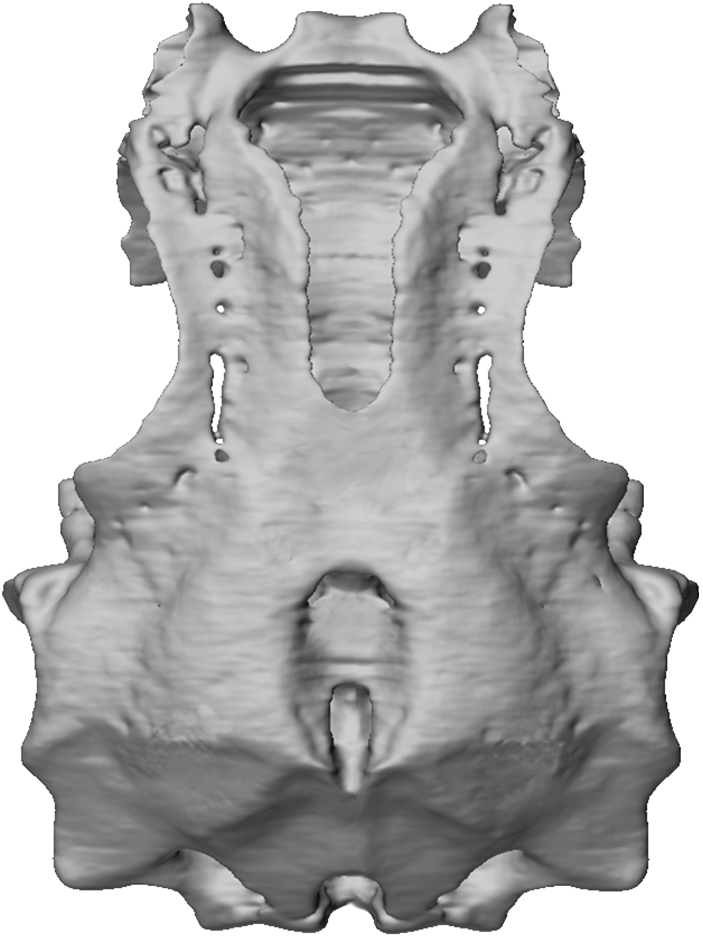
(1992), who reconstructed it as generally similar in overall morphology to that of Egertonodus basanus and other previously described hybodonts. However, that description was based on an incomplete specimen (lacking much of the ethmoid region and postorbital processes), the only material then available (fig. 2). Later, Maisey and Carvalho (1997) announced the discovery of a more complete specimen, AMNH 13958. Although this specimen (fig. 3) was mentioned in several later papers, revealing a selection of intriguing morphological details ( Maisey, 2004a, 2005, 2007), a complete description of its morphology has not been presented until now. Additional specimens of Tribodus limae neurocrania have since been discovered and are included in the present description (figs. 4, 5). CT scans of the braincase of AMNH 13958 were examined for the present study (figs. 6– 9), and were used to produce digitally rendered images of the braincase (figs. 10–14), cranial endocast (figs. 15–18), and skeletal labyrinth (figs. 19–22), described below.
Tribodus limae has a platybasic braincase (fig. 10), wedge shaped to trapezoidal in lateral view (fig. 13), in which the dorsal surfaces of the occipital and ethmoid regions slope downward to form obtuse angles with the top of the orbitotemporal region. When viewed under a light microscope, the pattern of prismatic calcified cartilage is visible on the surface of the neurocranium as a thin layer composed of dark brown tesserae, which appear rounded to polygonal in shape. The prismatic cartilage is particularly visible on the ventral surface of the braincase, and in the area surrounding the parietal (5 endolymphatic) fossa dorsally. This prismatic pattern is also visible in many of the other cartilaginous skeletal elements in the AMNH specimens of Tribodus , except those in which the surface was abraded during preparation (e.g., the jaws of AMNH FF 13958). As in other hybodonts and neoselachians (and unlike many Paleozoic sharks), most of the skeleton is covered with only a single layer of prismatic cartilage, which wraps around to cover both outer and inner surfaces including that of the endocranial space.
In dorsal view (fig. 10) the braincase is weakly triangular, with the otico-occipital region approximately 1.5 times wider than the ethmoid region. Although the distal parts of the postorbital processes (figs. 10–13: popr) are broken off, their lateral extent can be extrapolated based on the shape of the preserved portion of each process. Based on this, it can be concluded that the widest part of the braincase was across the otic region, rather than between the postorbital processes as in Egertonodus ( Maisey, 1983) . The maximum width of the braincase in Tribodus is 88.5 mm, 78% of its total length of 113 mm. As in Egertonodus ( Maisey, 1983) , the width of the neurocranium is only slightly less than its length.
The margins of the orbits form a more complete oval than in Egertonodus basanus ( Maisey, 1983) , in which the broad forwardpointed postorbital processes give the orbits a more anteriorly directed appearance. A major difference between Tribodus and Egertonodus is the lack in Tribodus of an anteroposterior ventral ethmoidal keel (the ‘‘caudal internasal keel’’; Maisey, 1983: figs. 9B–D: cik; this paper, figs. 29B, 33, 34: cik). Instead, the ventral surface of the ethmoid region in Tribodus is smooth and very slightly convex both anteroposteriorly and transversely, and slopes posteroventrally before joining with a median ventral process (fig. 11: mvpr), located between and directly posterior to the antorbital processes. Tribodus also lacks the inflated ethmopalatine processes of Egertonodus . A raised supraorbital crest is present above the orbits in Tribodus . At the widest part of the neurocranium (at the level of the otic capsules) are paired, rectangular, laterally concave structures, which are fibrous and lack prismatic calcification (fig. 3: csa). These have been identified as the points of articulation for the cephalic spines ( Maisey and Carvalho, 1997). The convexly curved cephalic spine bases fit neatly within these concave, bowl-shaped platforms, with the curved distal cusps of the spines directed posteriorly. Elevated ridges on the cranial roof immediately anterior and posterior to the parietal fossa correspond to the positions of the anterior and posterior semicircular canals (cf. Notorynchus ; Maisey, 2004b). As in Egertonodus basanus ( Maisey, 1983) and many other hybodonts, the otico-occipital region is ‘‘telescoped’’ forward between the postorbital processes, so that the otic capsules are situated between these processes (unlike many Paleozoic sharks, in which the otic capsules are located behind the postorbital processes). However, in Egertonodus the occiput projects somewhat farther posteriorly than it does in Tribodus and most neoselachians (cf. Maisey, 1983).
ROSTRAL AND ETHMOIDAL REGIONS
PROPORTIONS OF THE ETHMOID REGION: The ethmoid region of Tribodus limae is proportionally wider across than it is in Egertonodus basanus , and the olfactory capsules are more widely spaced. The left olfactory chamber of
AMNH 13958 is well preserved, and although its interior is filled with matrix, it can be observed via CT scanning (fig. 6A). The right olfactory chamber is not preserved. The ethmoid region of Tribodus comprises about one-third of the entire length of the braincase, unlike Egertonodus basanus and Hybodus reticulatus , in which the ethmoid region constitutes only one-quarter of its length ( Maisey 1983, 1987). Maisey (1983) describes the ethmoid region in Egertonodus as shorter than in most living sharks, but similar in length to that of Tristychius , while in Cobelodus , Tamiobatis , and Xenacanthus , the proportion is even lower ( Maisey, 2005, 2007). In the extant shark Notorynchus ( Maisey, 2004b) , the ethmoid region comprises approximately one-third of the total braincase length, resembling the condition in Tribodus . The proportional length of the ethmoid region in Tribodus thus more closely resembles that of some extant neoselachians than that of other hybodonts and outgroup chondrichthyan taxa.
PRECEREBRAL FONTANELLE, ROSTRAL BAR, AND BASICRANIAL PROCESS: The precerebral fontanelle of Tribodus limae is oval in cross section, and is slightly wider across than in Egertonodus basanus (fig. 3B, 6A–D). At the anteroventral tip of the precerebral fontanelle, the base of a forward-directed process is visible, although its anterior portion is missing (fig. 3B: rb). This is interpreted as a rostral bar, as in Egertonodus ( Maisey, 1983) . On the ventral medial surface of the rostral bar (fig. 11) there is a small, ventrally directed process, which appears V-shaped in frontal view. Posterior to this, the ventral surface of the internasal septum is broad and smooth, without a caudal internasal keel medially or paired ethmopalatine processes laterally such as those described in Egertonodus by Maisey (1983: fig.9: cik, ethppr).
Farther posteriorly, a large, medial, ventrally directed process is present on the ventral surface of the basicranium, originating anteriorly directly between the bases of the antorbital processes and extending posteriorly to just below the anterior margin of the optic foramen (figs. 3, 5, 6, 11: mvpr). This process is located immediately anterior to the anterior margin of the palatoquadrate, as illustrated by UERJ-PMB-114 (fig. 5B). This median ventral process was referred to as the basicranial process by Maisey and Carvalho (1997). It is possible that the basicranial process is homologous to the caudal internasal keel of other hybodonts, due to its ventromedial position and location in the anterior region of the braincase (also originating as an outgrowth of the embryonic trabeculae), and its probable similar involvement in jaw suspension (perhaps as an attachment point for soft tissues involved in jaw support in the case of Tribodus , rather than directly abutting the palatoquadrates as in Egertonodus ). In Egertonodus the caudal internasal keel extends from the tip of the rostrum to the anterior margin of the suborbital shelf. This includes the area where the ventral process is located in Tribodus , further suggesting that the two structures could be homologous. However, the basicranial process of Tribodus differs from the caudal internasal keel of Egertonodus in its much shorter anteroposterior extent. It is also possible that the median ventral process of Tribodus is homologous to a ventral interorbital septum, as in Squaliolus ( Maisey, 2007) , which like the process in Tribodus forms a solid wall of cartilage in the basicranium, and is located between the antorbital process and optic foramen.
The median basicranial process in Tribodus could alternatively be homologous to the keel process of some modern squalean sharks (e.g., Centroscyllium , Etmopterus ; Maisey, 2007), which also typically terminates immediately anterior to the palatoquadrates and is located between the optic foramen and antorbital process. In extant elasmobranchs, the preorbitalis muscle (5 suborbitalis of Shirai, 1992; Maisey, 2007) originates at the dorsal end of this process in Etmopterus and Centroscyllium . According to Maisey (2007), the interorbital septum of Squaliolus is probably not homologous to the keel process of the aforementioned sharks, because the latter forms later in ontogeny and differs in its ontogenetic point of origin. Because Tribodus is extinct and its ontogeny is unknown, the exact homology of its median ventral process to similar structures of modern forms remains uncertain. However, due to its location and probable function (confined to the anterior of the orbit between the antorbital processes and optic foramen; immediately anterior to palatoquadrate symphysis; not directly articulating with a forwardly extended palatoquadrate), it seems more likely that this process was homologous to the ventral orbital processes of modern elasmobranchs than to the caudal internasal keel of Egertonodus . A similar basicranial process occurs in embryos of the extant batoid Torpedo , but disappears later in ontogeny ( Holmgren, 1940: fig. 162: m.a.). This process was described by Holmgren (1940: 169) as a ‘‘distinct rudiment… corresponding to the posterior part of the medial area in Etmopterus and Squalus ,’’ and is positioned in front of the palatoquadrate symphysis. In Torpedo , this process is continuous with an anteroposteriorly elongated ridge or medial lamella (the ‘‘medial area’’ of Holmgren), which extends from nearly the anterior tip of the braincase to just in front of the palatoquadrate, but later disappears. Interestingly, this elongated ridge covers the same area as the caudal internasal keel of Egertonodus . The last part of the ridge to remain during ontogeny is the small, anteroposteriorly compressed median process (the ‘‘rudiment’’ described above), which superficially resembles the median ventral process of Tribodus . Whether the ‘‘medial area’’ of Torpedo is homologous to the median ventral process of Tribodus , and/or the caudal internasal keel of other hybodonts, is uncertain. However, it suggests that the two structures found in hybodonts could be homologues of each other, resulting from similar ontogenetic processes.
As in Egertonodus and Chlamydoselachus , the anteroventral margin of the precerebral fontanelle in Tribodus is defined by the slightly upturned forward part of the internasal septum ( Maisey, 1983). The precerebral fontanelle is infilled with matrix, making structures on the interior walls and floor of the fontanelle difficult to examine; however, these structures can be observed with the aid of CT scanning (fig. 6A–D). In a three-dimensional image of the braincase made from CT scans of AMNH 13958, the olfactory nerve foramen is visible as a large opening in the internasal septum connecting the precerebral fontanelle with the olfactory chamber (fig. 14, I). The olfactory nerve canal is visible on the interior of the braincase as an elongated furrow on the ventrolateral wall of the ethmoid region (fig. 14: olf can).
VASCULARIZATION AND INNERVATION OF THE ETHMOID REGION: Allis (1923) and Jarvik (1942) noted three major groups of nerves and blood vessels in the anterior of the orbit in Chlamydoselachus : a set of dorsal and ventral foramina within the orbit, and an additional group of structures that pass superficially, lateral to the ectethmoid process ( Maisey, 1983). These include the maxillary artery, the facial vein, and two nerve rami: the maxillary ramus of the trigeminal nerve, and the buccal ramus of the facial nerve (now revised as the buccal ramus of the anterodorsal lateral line nerve; Northcutt and Bemis, 1993). Based on comparison with modern elasmobranchs such as Chlamydoselachus, Maisey (1983) provided an interpretation of the innervation and vascularization of the ethmoid region of Egertonodus basanus . Based on this interpretation and on CT scan images of Tribodus limae (AMNH 13958), small, paired foramina on the ventral surface of the internasal lamina (located directly posterior to the nasal capsules and opening into them) are interpreted as anterior openings for the orbitonasal vein canals (figs. 3A, 6B, 11: onc). These connect internally with corresponding small, ventrally located openings in the anterior of each orbit, where the orbitonasal veins presumably exited the orbit (figs. 2C, 6B–F, 11, 13: onc). The positions of these foramina for the orbitonasal vein are consistent with those described in Egertonodus and other elasmobranchs (e.g., Chlamydoselachus and Notorynchus ; Maisey, 2004b, 1983; Shirai, 1992; Allis, 1923). Allis (1923) described the orbitonasal vein in Chlamydoselachus as originating in the nasal capsule and traversing the ectethmoid chamber before entering the orbit via the orbitonasal foramen. The positions of the orbitonasal foramina in Tribodus are consistent with this description. Based on the inferred position of the orbitonasal canal, the part of the postnasal wall lateral to the orbitonasal foramen in Tribodus limae is then the planum antorbitale, or antorbital process (figs. 3A, C, 10–13: ant), originating from the lamina orbitonasalis of the embryonic trabecula, according to the interpretation given by De Beer (1931).
On the ventromedial surfaces of the antorbital processes of AMNH 13958 are small, paired, rounded structures, immediately lateral to a large notch separating the distal end of the antorbital process from the interorbital wall (figs. 3A, 11, 12: pr.art). The rounded shape of these paired structures, together with their lack of prismatic cartilage distally, indicate that they may have served as an attachment point for muscles or ligaments involved in jaw suspension. There are several examples of extant elasmobranchs in which the antorbital processes serve as muscle attachment sites: the suborbitalis (5 preorbitalis, Maisey, 2007) muscle in Chlamydoselachus , hexanchoids, and the squalean Echinorhinus originates at the tip of the antorbital process, and the adductor mandibularis superficialis muscle in hexanchoids inserts on the antorbital process as well as behind the eye ( Shirai, 1992). In extant carcharhinoids, the ventral head of the preorbital (5 suborbitalis, Maisey, 2007) muscle originates in the anterior of the orbit or on the posterior of the nasal capsule ( Compagno, 1988: fig. 8.1). Interestingly, small rounded Fig. 10 View Fig .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |