Knipowitschia caunosi, Ahnelt, Harald, 2011
|
publication ID |
https://doi.org/ 10.5281/zenodo.202003 |
|
DOI |
https://doi.org/10.5281/zenodo.6189182 |
|
persistent identifier |
https://treatment.plazi.org/id/A02DA27D-FFB6-FFB6-FF7A-41AF98E3FE97 |
|
treatment provided by |
Plazi |
|
scientific name |
Knipowitschia caunosi |
| status |
sp. nov. |
Knipowitschia caunosi View in CoL sp. nov.
Caunos Goby
Figures 1 View FIGURE 1 , 3 View FIGURE 3
Synonymy. Pomatoschistus (Bubyr) caucasicus kosswigi . Ladiges 1964: 212 (part).
Holotype. ZMH 2175:16, female, 26.0 mm SL; Turkey, Lake Köycegiz, 36°55’N, 28°40’E; collected 31. October 1946.
Paratypes. ZMH 2175:17, one male, 25.6 mm SL, ZMH 2175:18, one female, 26.6 mm SL; same as holotype.
Etymology. Named for the mythological figure Caunos, twin brother of Byblis and founder of the ancient Carian city Caunos. The ruins of this city are located close to Lake Köycegiz.
Diagnosis. A Knipowitschia with (1) scales from the axilla in midline to the caudal peduncle, extending onto base of caudal fin; (2) anterior oculoscapular canal present, ending anteriorly with a pair of interorbital pores; (3) preopercular canal present with two terminal pores; (4) external preorbital neuromast row extending anteriorly close to the upper lip; (5) anterior preorbital row absent; (6) anterior transverse row long, extending on the nape; (7) pelvic frenum with smooth free edge; (8) male colouration without vertical bars or striae, dark blotch in the rear of the first dorsal fin; first dorsal fin rays V–VI.
Description. Measurements of the holotype (25.9 mm SL) and two paratypes (25.6–26.5 mm SL) as percent of standard length (range and mean in parentheses, values of holotype in brackets): head length 25.7–25.8 (25.8) [25.5], head width 11.3–12.5 (11.9) [11.9], distance from snout to origin of first dorsal fin 36.2–37.5 (36.9) [37.6], distance from snout to origin of second dorsal fin 54.7–55.8 (55.3) [55.2], distance from snout to anus 50.8–55.5 (53.2) [54.4], distance from snout to origin of anal fin 55.5–59.2 (57.4) [58.3], distance from snout to origin of pelvic fin 29.3-31-3 (30.3) [29.7], caudal peduncle length 28.3–29.7 (29.0) [29.7], length of first dorsal fin base 8.7+10.7 (9.7) [8.9], length of second dorsal fin base 14.7–16.8 (15.8) [15.1], length of anal fin base 12.9–14.4 (13.7) [12.4], caudal fin length 20.4–22.3 (21.4) [damaged], pectoral fin length 20.0–22.3 (21.2) [20.1], pelvic fin length 22.3+23.0 (22.7) [22.0], body depth at pelvic fin origin 19.6–19.9 (19.8) [19.3], body depth at anal fin origin 15.5–16.8 (16.2) [16.2], body width at anal fin origin 9.8–10.2 (10.0) [10.0], caudal peduncle depth 9.0–9.8 (9.4) [9.3], distance from origin of pelvic fin to anus 23.8–26.4 (25.1) [28.0]; as percent in caudal peduncle length: caudal peduncle depth 32.0–32.9 (32.5) [31.2]; as percent of head length: snout length 24.2–26.5 (25.4) [22.9], postorbital length 46.6–55.1 (50.9) [51.5], eye diameter 24.2–27.9 (26.1) [22.7], cheek depth 20.6–22.7 (21.7) [17.7]; as percent of eye diameter: interorbital width 47.4–50.0 (48.7) [53.3]; as percent of distance from origin of pelvic fin to anus: pelvic fin length 85.7–95.2 (90.4) [82.9].
Counts (values of the holotype are indicated by *): first dorsal fin V–VI (V: 2, VI*: 1); second dorsal fin D2 I/ 8; A I/7–8 (7: 1, 8*: 29); pectoral fin 15–17 (15*: 2, 17: 1); pelvic fin complete, pelvic fins (united, forming disc) I/ 5 + 5/I, fifth ray longest; caudal fin 15 segmented and 13 branched rays; scales in lateral series 30 –32 (30: 1, 32*: 2), scales in transversal series 8.
The body squamation is typical for a Knipowitschia species with head, nape, back to origin of second dorsal fin and breast naked. The ctenoid scales extend posteriorly from the base of the pectoral fin on the base of the caudal fin.
Lateral-line system ( Fig. 3 View FIGURE 3 ): The anterior oculoscapular canals are fused in the midline at a single pore D with paired interorbital pores C and postorbital pores F and H or not fused in midline with two pores in position of pore D and with short remnants of the interorbital canals anterior to pore D, as a short canal or as furrow. A preopercular canal is developed, with pores M and O, in one specimen or just as an open furrow in the other two.
Rows and, in parentheses, mean of numbers of free neuromasts (sensory papillae) from the three type specimens which are important to separate this species from K. byblisia and from other congeners.
Innervation by the antertior lateral-line nerve: (1) supraorbital: longitudinal rostral row s (10.3) a continuous row ending anteriorly close to upper lip; longitudinal interorbital row p absent, also in one specimen where the interorbital section of the supraorbital canal is not closed but present as a deep furrow. (2) Infraorbital: longitudinal row a (8.3) with two to three short transverse rows; transversal row s3 absent. (3) Hyomandibular longitudinal row b (11.7) anteriorly reaching below orbit; longitudinal row i (32.3) ending close to pore O of the preopercular canal. (4) Otic transversal row tra (6.6) confluent with supraorbital row n and ventrally extending two third of distance to hyomandibular longitudinal row b.
Innervation by the posterior lateral-line nerve: (1) supratemporal transversal row tr (13.5) long, reaching close to longitudinal row m (5.3). (2) Posterior dorsal row y absent. Posterior lateral transverse axillary row as1 (8) short, dorsally not extending above the longitudinal axillary rows la.
Coloration in preserved specimens. Description of colouration is based on preserved material. All specimens are pale fawn. No markings on the body have been identified. The first dorsal fin in males has a distinct dark spot in the rear, extending from fifth ray to the end of the fin membrane. No dark blotch on chin in females.
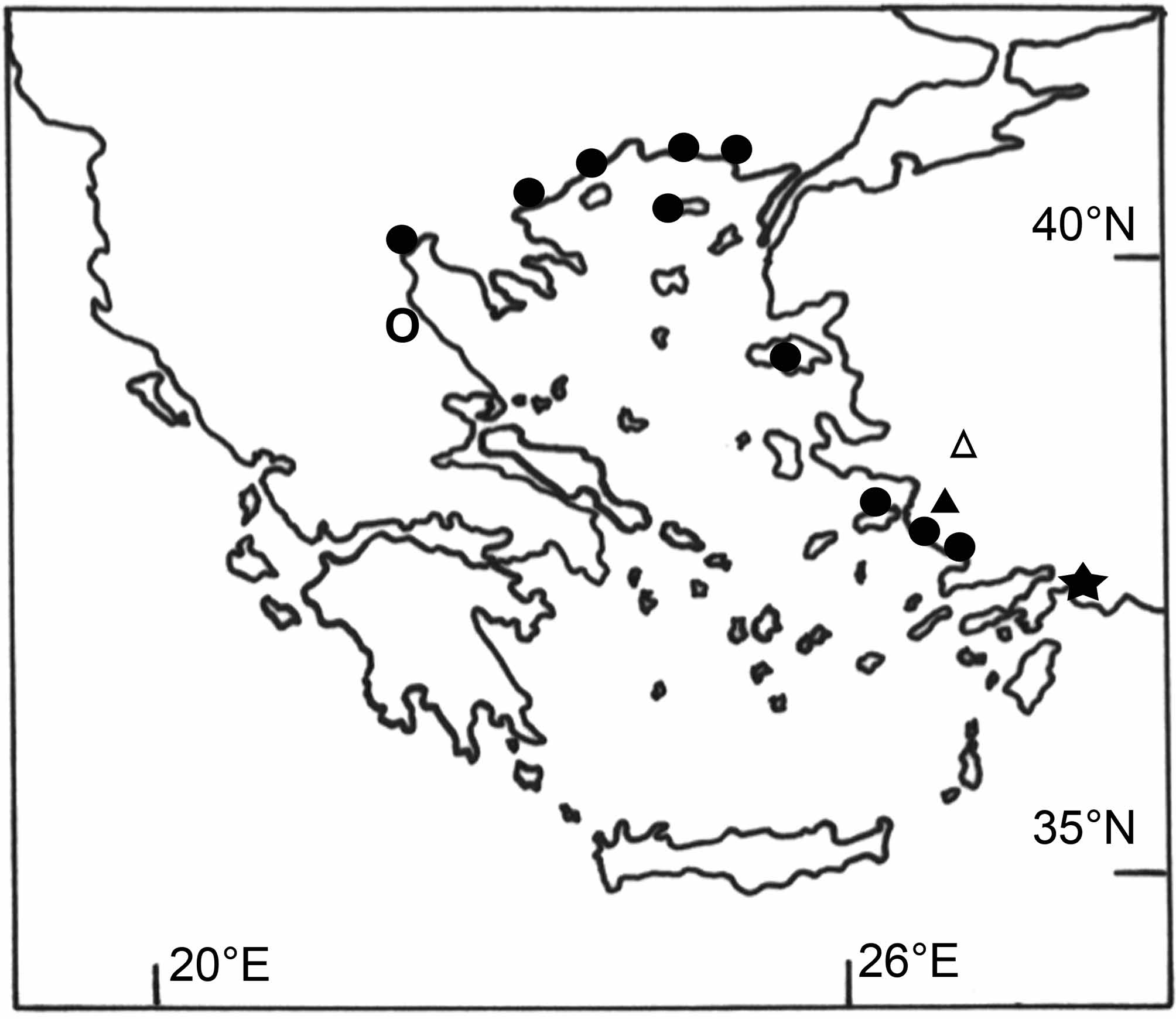
Remarks. Distribution: Six species of the Ponto-Caspian genus Knipowitschia , K. caucasica , K. ephesi , K. mermere , K. thessala (Vinciguerra) , K. byblisia sp. nov. and K. caunosi sp. nov. occur in peripheral habitats around the Aegean Sea (Ahnelt et al. 1995; Miller 2004; this study) ( Figure 4 View FIGURE 4 ). These species are primary-like freshwater fishes that evolved from an ancient euryhaline ancestor of Paratethys and show a similar pattern of distribution to that of primary freshwater fishes ( Bianco et al. 1996). They likely dispersed the same way, were affected by connection events during the last glacial maxima, and may have found refuges in suitable coastal habitats after sea level rises (Ahnelt 1995; Bianco et al. 1996; Durand et al. 2003; Reyjol et al. 2007).
Knipowitschia byblisia View in CoL and K. cauonsi , only known from Lake Köycegiz, represent the southern and easternmost autochthonous record of this genus in the Mediterranean region and the first record of Knipowitschia View in CoL in the transition of the Aegean to the eastern Mediterranean. Note that K. caucasica View in CoL has been introduced unwittingly to Anatolian lakes ( Van Neer et al. 1999). Recently, K. caucasica View in CoL has also been reported from Lake Köycegiz ( Balik et al. 2005). Possibly this species has been misidentified (see Ladiges 1964), or K. caucasica View in CoL has been introduced with fry of Cyprinus carpio Linnaeus View in CoL , as has been previously described for Anatolian lakes ( Van Neer et al. 1999). All carp fry stocked before 1995 came from fish farms at the River Evros/Meric (Thracia), where K. caucasica View in CoL occurs naturally ( Van Neer et al. 1999). Furthermore, a land-based fish farm, a possible source of introduction, is located close to the Köycegiz tributary Yuvarlakcay ( Taseli 2009). Stocking of C. carpio View in CoL for recreational fishing started in the 1950s/1960s (Innal & Erk’akan 2006). Nevertheless, the material under investigation was collected in 1946, which confirms the autochthonous origin of K. byblisia View in CoL and K. caunosi in Lake Köycegiz.
Ecology. No ecological data are associated with the sample of Knipowitschia View in CoL from Lake Köycegiz and the exact collection site is unknown. This small lake is characterised by brackish water ( Akin et al. 2005), which would have favoured a euryhaline stock of Knipowitschia View in CoL . Lake Köycegiz, a former bay of the Mediterranean Sea, was already in existence 4,000 BP. About 2,000 BP the harbour of the ancient city of Kaunos, located close to the lake, lost its connection to the open sea ( Brückner 1997).
Origin. The occurrence of K. byblisia View in CoL and K. caunosi in the transition area from the Aegean region to the eastern Mediterranean can be explained by two major events: the Messinian salinity crisis of the Mediterranean Sea at the end of the Miocene (about 5.6 million years ago) and the Pleistocene glaciations (between 650,000–11,000 years ago). During the latter event, the salinity of the Black Sea varied substantially ( Yanko-Hombach et al. 2007). At the end of the glacial maxima outflow of low salinity (2–6 ppt) or even fresh water connected the Black Sea with the Mediterranean Sea (summarized in Yanko-Hombach et al. 2007). This enabled immigration of the autochthonous Ponto-Caspian ‘sand goby’ genus Knipowitschia View in CoL , in particular the euryhaline K. caucasica sensu Miller (2004) View in CoL to the Aegean region where this species inhabits circum Aegean lagoons and freshwater habitats ( Economidis & Miller 1990; Ahnelt et al. 1995, Bianco et al. 1996). Gene flow between these populations is limited or prevented by marine conditions (33 ppt) and by the counter-clockwise surface currents in the Aegean Sea ( Hamad et al. 2005). A similar distribution in isolated freshwater habitats in the Mediterranean basins is known from Gasterosteus aculeatus Linnaeus. These View in CoL populations apparently trace back to a stock of G. a c u l e a t u s which inhabited the Mediterranean Sea during Pleistocene glacial periods ( Paepke 2002).
The second event, the salinity crisis of the Mediterranean, is characterized by a drastic drop in sea level and subsequently the establishment of brackish conditions in the “Lago Mare” phase of the Mediterranean basins ( Penzo et al. 1998; Huyse et al. 2004). The brackish fauna included many species of Paratethys origin ( Popov et al. 2006) reaching the Mediterranean basins by spill-over ( Rögl 1998). These fresh and brackish environments enabled the dispersal of euryhaline and freshwater fishes ( Reyjol et al. 2007). After the formation of the modern Mediterranean Sea about 5.3 million years ago only isolated populations of a euryhaline ancestor of the Mediterranean Knipowitschia View in CoL populations survived in restricted areas such as estuaries and brackish lagoons ( Penzo et al. 1998; Huyse et al. 2004). Possibly such an ancestor gave rise to the Adriatic and the Aegean Knipowitschia View in CoL stocks, and isolation in estuaries, brackish lagoons and peripheral freshwaters clearly favoured speciation. Similar relicts of the Mediterranean ‘Lago Mare’ fish fauna are the recent Aphanius View in CoL and Salaria View in CoL species ( Perdices et al. 2000; Hrbek & Meyer 2003).
Knipowitschia byblisia View in CoL and K. caunosi occur sympatrically in a small coastal lake. Such a sympatric occurrence of two closely related Knipowitschia View in CoL species is rare ( Miller 2004), but is possibly based on different radiation scenarios. K. byblisia View in CoL , the most specialized and in its paedomorphic features similar to K. ephesi View in CoL and K. mermere View in CoL from eastern Aegean freshwater habitats (see below), could trace back to an ancient Knipowitschia View in CoL stock from the Mediterranean ‘Lago Mare’ phase. K. caunosi , more similar to K. caucasica View in CoL , apperently radiated from a K. caucasica View in CoL stock reaching the area of the Köycegiz lake during melting phases of Pleistocene glaciations. Possibly these different radiation scenarios also explain the paraphyletic position of the genus Knipowitschia View in CoL proposed by Penzo et al. (1998) and Hyuse et al. (2004).
Morphology. Knipowitschia caunosi View in CoL , and to a lesser extent K. byblisia View in CoL , resemble the euryhaline eastern Aegean K. caucasica View in CoL stock but differ in squamation and in the type of reduction of the head canals. Differences are distinct, particularly with K. caucasica View in CoL , a species of larger body size and with completely developed head canals of the lateral line system, with fusion of the anterior oculoscapular canals in midline and posterior oculoscapular canal present ( Miller 2004). With reduction of body size, in head lateral-line canals and squamation, K. byblisia View in CoL shows similar paedomorphic features to K. ephesi View in CoL and K. mermere View in CoL (Ahnelt 1995; Ahnelt et al. 1995) or the freshwater species from the Adriatic region ( Kovacic 2005; Kovacic & Sanda 2007). All these species are miniature as adults (<28 mm SL) and characterised by high morphological uniformity due to paedomorphosis. Small body size offers increased habitat opportunities, a fine subdivision of the environment and it enables survival in small habitats with limited or fluctuating resources ( Dial & Marzluff 1988; Miller 1996). Additionally, small body size is often linked to short generation time ( Miller 1996), which favours diversity and speciation ( Martin & Palumbi 1993).
Completion of the life cycles of small gobiid fishes, in isolated and small habitats seems to favour reduction or loss of squamation and of the head lateral-line canals (Ahnelt 1995; Ahnelt et al. 2004; Kovacic & Sanda 2007). If replaced by free neuromasts, the loss of head canals may also contribute to increased sensitivity of the lateral line system as discussed for Eucyclogobius newberryi (Girard) View in CoL (Ahnelt et a l. 2004; Earl et al. 2010). This small northeast Pacific gobiid shows similar reductions of the supraorbital canal to K. byblisia View in CoL (Ahnelt et al. 2004, Fig. 3 View FIGURE 3 ). Clearly similar habitat and life history, combined with low dispersal, favour similar morphological characteristics, such as small body size, reduced squamation and reduced head lateral-line canals. Such scenarios apparently occurred independently in E. newberryi View in CoL and in the Adriatic and Aegean clades of Knipowitschia View in CoL . It is therefore likely that such a suite of characteristics is a general indicator of gobiid species adapted to small habitats and therefore of special interest for evolutionary studies and for conservation.
Conservation. The fresh water fish fauna of continental western Anatolia is critically endangered in coastal areas by anthropogenic effects (Ahnelt et al. 1995; Innal & Erk’akan 2006). Agriculture, aquaculture, hydro power stations and tourism greatly affect the habitats of fresh water fishes. The native fish fauna of Lake Köycegiz is affected by pollution and increasing tourism, as well as by the introduction of non-native fish species (Innal & Erk’akan 2006, Taseli 2009). There is also a single record of K. caucasica View in CoL in Lake Köycegiz ( Balik et al. 2005). If this is correct, this congener, with similar ecological demands and larger body size, could out-compete the two native species. Additionally, introducing alien species, e.g. Tilapia zillii (Gervais 1848) View in CoL ( Akin et al. 2005; Balik et al. 2005) will increase predation pressure. Known only from Lake Köycegiz, it is likely that K. byblisia View in CoL and K. caunosi View in CoL are threatened by competition and predation pressure imposed by introduced species.
| ZMH |
Zoologisches Museum Hamburg |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Knipowitschia caunosi
| Ahnelt, Harald 2011 |
K. caucasica sensu
| Miller 2004 |
Tilapia zillii
| Gervais 1848 |