Losdolobus nelsoni, Pompozzi, Gabriel, 2015
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3990.3.6 |
|
publication LSID |
lsid:zoobank.org:pub:B3FAC2CE-B3A6-4BB3-9892-EB70CCA13A8D |
|
DOI |
https://doi.org/10.5281/zenodo.6112518 |
|
persistent identifier |
https://treatment.plazi.org/id/B23387FE-1C2E-951A-299B-EA823865FD45 |
|
treatment provided by |
Plazi |
|
scientific name |
Losdolobus nelsoni |
| status |
sp. nov. |
Losdolobus nelsoni View in CoL sp. nov.
Figs 1–9 View FIGURE 1 View FIGURES 2 – 4 View FIGURES 5 – 6 View FIGURE 7 View FIGURE 8 A – B View FIGURE 9
Types. Male holotype (MACN-Ar N°34452) and females paratype (MACN-Ar 34453; MACN-Ar 34454) from Reserva Natural “Sierra del Tigre” (37°22´46´´S – 59°7´44´´W), Tandil, Buenos Aires Province, Argentina, July 2011, N. Ferretti leg.
Etymology. The specific epithet is a patronymic in honor of the collector of the specimens, the arachnologist Dr. Nelson Ferretti.
Diagnosis. The male of L. nelsoni sp. nov. differs from congeners by having an enlarged embolus with a slight curvature. The bulb resembles that of L. opytapora and L. parana but differs by having a noticeably longer embolus; it differs from that of L. xaruanus by having the base of the embolus at a more acute angle relative to the tegulum ( Fig. 4 View FIGURES 2 – 4 ). The female differs from L. opytapora by the presence of a straight median rod and inconspicuous circular secretory plate, and from L. parana by not having the tibia of the palp inflated ( Fig. 8 View FIGURE 8 A – B ). The female genitalia of L. nelsoni sp. nov. resembles that of L. xaruanus but differs by having a thicker median rod.
Description. Male (holotype, MACN-Ar N°34452). Carapace, labium, endites and sternum yellow. Eyes on black pigment. Chelicerae yellow, fang fulvous dark orange. Legs yellow. Abdomen pale-yellow ( Fig. 1 View FIGURE 1 ). Total length 1.91. Carapace oval 0.86 long, 0.69 wide. Chelicerae 0.40 long, 0.20 wide. Labium triangular 0.14 long, 0.14 wide as it base. Endites 0.25 long, 0.25 wide. Sternum slightly wider than long. Abdomen 1.05 long, 0.90 wide, 0.65 high. Eye diameters and interdistances: ALE 0.08 long, 0.05 wide, PME 0.09 long, 0.06 wide, PLE 0.09 long, 0.08 wide, PME-PME 0.02, PME-PLE 0.06, PME-ALE 0.03, PLE-PLE 0.27, ALE-PLE touching. Leg spination: III tibia d0, p0, r0-1-1, v0, metatarsus d1-0-1, p0-0-1, r0-0-1, v0-0-1; IV tibia d0, p1-0-1, r1-0-1, v1 -0-1, metatarsus d1-1-1, p1-0-0, r1-0-1, v1 -0-0. Leg measurements: I femur 0.80/patella 0.37/tibia 0.82/metatarsus 0.45/ tarsus 0.25/total 2.69; II 0.75/0.34/0.71/0.57/0.28/2.65; III 0.60/0.23/0.60/0.48/0.25/2.16; IV 0.84/0.25/0.74/0.82/ 0.34/2.99. Palpal femur 0.37/patella 0.17/tibia 0.34/cymbium 0.20/bulb plus embolus 0.34. Palpal bulb simple and oval, longer than cymbium ( Figures 2–6 View FIGURES 2 – 4 View FIGURES 5 – 6 ).
Female (paratype, MACN-Ar 34453). Coloration as in male ( Fig. 7 View FIGURE 7 ). Total length 2.82. Carapace oval 1.20 long, 0.80 wide. Chelicerae 0.35 long, 0.15 wide. Labium triangular 0.12 long, 0.18 wide as it base. Endites 0.29 long, 0.15 wide. Sternum slightly wider than long. Abdomen 1.62 long, 1.10 wide, 1.05 high. Eye diameters and interdistances: ALE 0.08 long, 0.05 wide, PME 0.08 long, 0.06 wide, PLE 0.06 long, 0.04 wide, PME-PME 0.02, PME-PLE 0.07, PME-ALE 0.02, PLE-PLE 0.26, ALE-PLE touching. Leg spination: I femur d0, p0, r0, v1, tibia d0, p0, r0, v1; III tibia d1-0-0, p1-1-0, r1-0-0, v1 -0-0, metatarsus d1-0-0, p1-1-0, r0-0-1, v1 -0-0; IV tibia d0, p1-0- 0, r1-0-0, v1 -0-0, metatarsus d0, p1-1-1, r1-1-1, v0-1-1. Leg measurements: I femur 1.47/patella 0.53/tibia 1.13/ metatarsus 1.0/tarsus 0.4/total 4.53; II 1.13/0.53/1.06/0.96/0.43/4.11; III 0.93/0.36/0.60/0.56/0.36/2.81; IV 1.57/ 0.43/1.07/0.90/0.27/4.24. Palpal femur 0.47/patella 0.23/tibia 0.30/tarsus 0.43/total 1.43. Internal genitalia with median rod not bifid at tip and central process sinuous, circular secretory plate medially positioned, posterior receptaculum rounded, covered with bands of gland pores intercalated by smooth areas ( Figure 9 View FIGURE 9 ).
Other material examined. Argentina: Buenos Aires: General Pueyrredón: Mar del Plata, 20/ 21 July 1984, M. Ramírez, 1 male (MACN-Ar 20187); Tornquist: Parque Provincial Ernesto Tornquist (38°3´21.5″S– 61°58´87″W), October 2009, N. Ferretti, 2 males (LZI 0356), 2 females (LZI 0355); same, May 2010, N. Ferretti, 2 males (LZI 0352); same, June 2010, N. Ferretti, 1 male (LZI 0354), 1 female (LZI 0353); same, July 2010, N. Ferretti, 5 females (LZI 0351); Tornquist: Estancia Funke (38°4′20.40″ S, 62°3′8.12″ W), May 2012, N. Ferretti, G. Pompozzi, S. Copperi & L. Schwerdt, 1 male (LZI 369); same, November 2012, N. Ferretti, G. Pompozzi, S. Copperi & L. Schwerdt, 1 female (LZI 370); Tandil: Reserva Municipal Sierra del Tigre (37°22´46″S–59°7´44″W), June 2011, N. Ferretti, 3 males (LZI 0359), 13 females (LZI 0360); same, July 2011, N. Ferretti, 5 males (LZI 0365), 6 females (LZI 0366); same, August 2011, N. Ferretti, 10 males (LZI 0363), 12 females (LZI 0364); same, September 2011, N. Ferretti, 7 males (LZI 0361), 16 females (LZI 0362); same, October 2011, N. Ferretti, 1 female (LZI 0358); same, Apr 2012, N. Ferretti, 2 males (LZI 0357); Tandil, May 1967, Maury, 1 male (MACN-Ar 20186).
Distribution. Known from the mountain ranges of southwestern and central Buenos Aires Province (Ventania and Tandilia), Argentina ( Figure 10 View FIGURE 10 ).
Natural History. The Sierra del Tigre Municipal Reserve is in the Tandilia mountain range, southeast of Buenos Aires Province, Argentina. The reserve has an area of 142 ha and reaches a maximum altitude of 350– 450 m.a.s.l. ( Figure 11 View FIGURES 11 – 12 ). The climate is humid and temperate with an average annual rainfall of 900 mm ( Velázquez et al. 1998). The Ernesto Tornquist Provincial Park (ETPP) is located within the Ventania mountain range, and has an area of approximately 6700 ha. The topography ranges from steep slopes at high elevations of the range to gentler slopes at lower levels (piedmont) ( Figure 12 View FIGURES 11 – 12 ). This mountain range is characterized by its height: 800 – 900 m.a.s.l., with a few summits of up to 1240 m.a.s.l. ( Demoulin et al. 2005). The climate is humid and temperate with an average annual rainfall of 850 mm ( Pérez & Frangi 2000). The natural vegetation consists of more than 400 native species with high endemism. The Funke ranch is located next to the ETPP, and has similar conditions. This ranch has some degree of disturbance due to grazing by domestic livestock and sunflower and corn cultivars at the base of the hills. All of these places belong to the Biogeographic Province of Pampa ( Morrone 2001). All three locations are characterized by flat grasslands interspersed with mountains, with different degrees of disturbance due to urbanization, tourism, agriculture and livestock. These areas are remarkably different from those where L. parana , L. opytapora and L. ybypora have been reported. These species were collected in areas characterized by the presence of Araucaria angustifolia (Bert) O. Kuntze (Araucariaceae) , the Brazilian pine tree. The habitat of L. nelsoni sp. nov. is more similar to the habitat of L. xaruanus ( Lise & Almeida 2006) . In ETPP, Losdolobus nelsoni sp. nov. was found living in sympatry with two species of Oonopidae endemic to the mountain ranges of Buenos Aires: Puan chechehet Izquierdo 2012 and P. nair Izquierdo 2012 ( Izquierdo et al. 2012) . However, in Sierra del Tigre (Tandil) no specimens of Puan were reported.
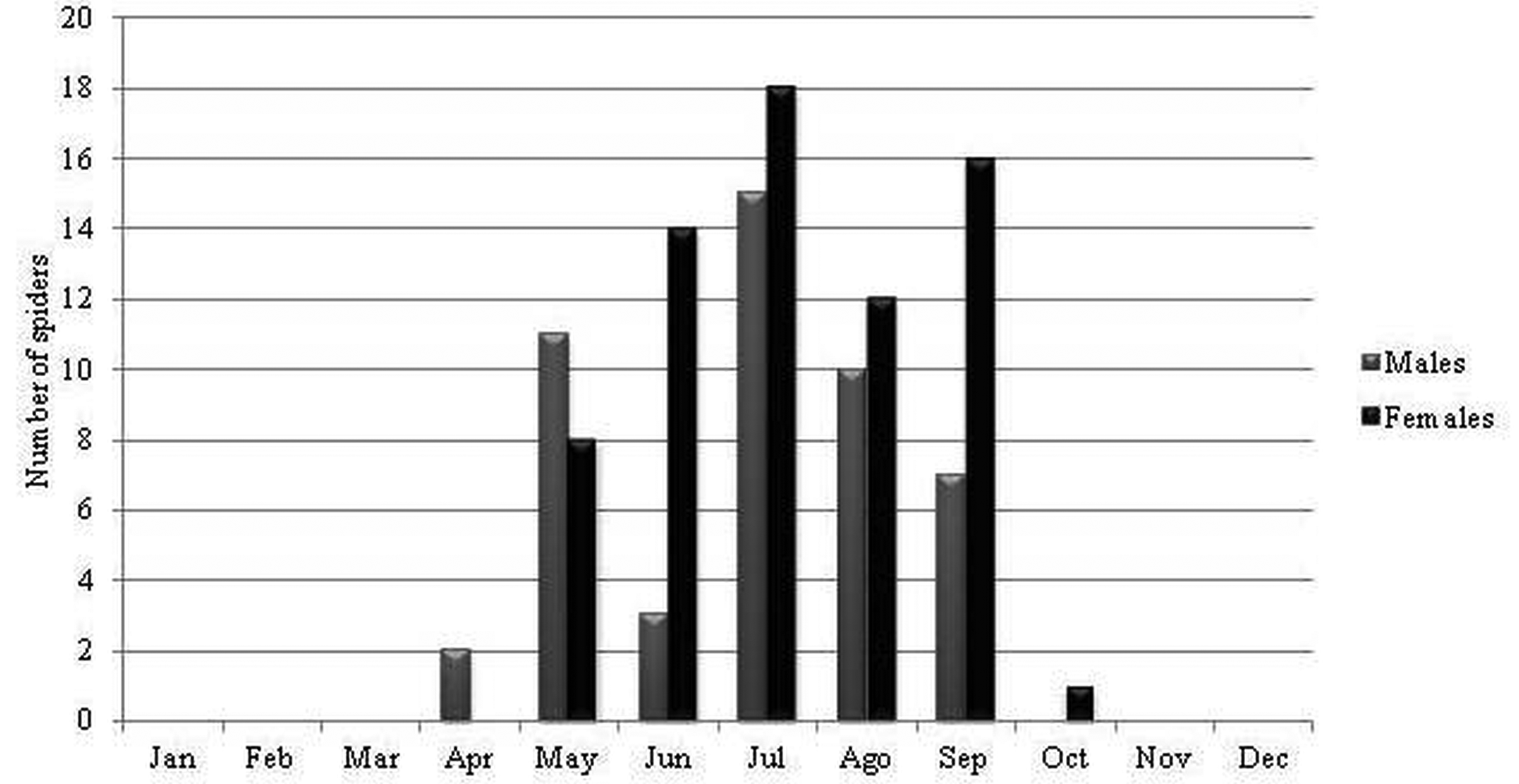
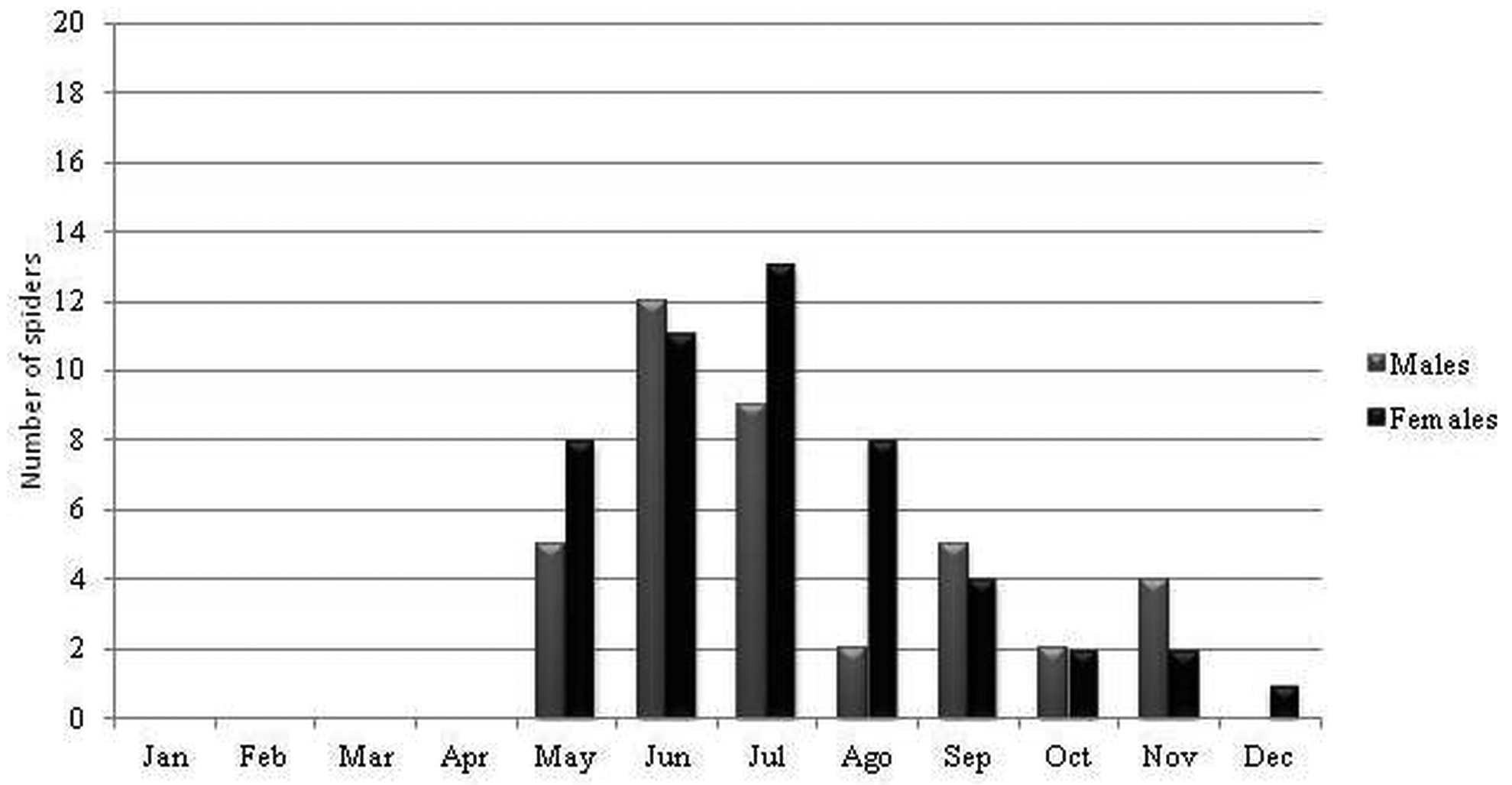
Ecology and biogeography. Losdolobus nelsoni sp. nov. has stenochronous adults (present during only a short period of time during the year) because the majority of the adults were found between May and September (coldest months in southern hemisphere) ( Figures 13–14 View FIGURE 13 View FIGURE 14 ). This species has non-overlapping generations and a winter mating period. Stenochronous species are phenological ‘‘specialists’’ adapted to completing various life history stages at specific times when conditions are most favorable ( Draney & Crossley 1999). According to Draney & Crossley (1999) this kind of strategy may be useful to avoid potential predators that show low frequencies and activity during winter. In STMR males were observed from May to September, and the highest activity for males was recorded during July and August ( Figure 13 View FIGURE 13 ), whereas for males in ETPP the highest activity was during June and July, and males were observed from May to November ( Figure 14 View FIGURE 14 ). Females were most abundant during the same period with the exception of one female recorded in October in STMR, and one female recorded in December in ETPP ( Figures 13–14 View FIGURE 13 View FIGURE 14 ). This cycle is similar to that found for L. opytapora and L. ybypora . The males of these two species were found between May and September ( Brescovit et al. 2004). In addition, the only male of L. parana that has been found was captured in August ( Platnick & Brescovit 1994), confirming a winter reproductive period for the genus. Curiously, juveniles were not found in any month of the sampling, similar to what has been reported for Puan species in ETPP ( Izquierdo et al. 2012).
The family Orsolobidae presents a gondwanic distribution, where the greatest number of species has been described for New Zealand and Chile as well as some other regions of South America, Australia and South Africa (World Spider Catalog 2015). Due to this southern distribution, Platnick & Brescovit (1994) were surprised to find specimens of an orsolobid species from southern Brazil. Then, three additional species were described for the southern region of Brazil ( Brescovit et al. 2004; Lise & Almeida 2006). However, when analyzing the distribution localities of Losdolobus species I found an interesting pattern that follows the ‘peripampasic arc’ ( Figure 10 View FIGURE 10 ). The peripampasic arc comprises a group of mountain ranges from Argentina, Uruguay and southern Brazil ( Frenguelli 1950; Acosta 1993; Mattoni & Acosta 1997) ( Figure 10 View FIGURE 10 ). The peripampasic arc is biogeographically interesting.
These mountain ranges have many endemic species ( Crisci et al. 2001; Grela 2004; Pinto-da-Rocha et al. 2005; Aagesen et al. 2009) and are considered important for biodiversity conservation ( Szumik et al. 2007; Navarro et al. 2009; Nori et al. 2011; Ferretti et al. 2012a, Simó et al. 2014). The distributional patterns of Asteraceae ( Crisci et al. 2001) showed a connection among Tandilia and Ventania and southern Brazil, Pampa, Uruguay and Pampean ranges, although the authors proposed that Tandilia is closer to Uruguay and southern Brazil than to Ventania. Although the geological evidence suggests that both mountain ranges resulted from independent geological processes at different geological times, they are geographically close to each other ( Ferretti et al. 2012a). The distribution of the genus Losdolobus could be explained by the peripampasic arc, however, more studies are needed to confirm this hypothesis. For example, exhaustive sampling in mountain ranges from eastern Uruguay and central Argentina, areas where the perimpampasic arc hypothesis predicts Losdolobus occurrence, could confirm this type of distribution. The only record for L. parana reported for Argentina ( Grismado & Izquierdo, 2014), collected in Mar del Plata, is a misidentification and actually corresponds to L. nelsoni sp. nov., thus there is only one species of Losdolobus known in Argentina. More studies are needed in this area to reveal the limits of the distribution of the genus Losdolobus in Argentina.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.