Microlympia echina, Shear, William A. & Leonard, William P., 2003
|
publication ID |
https://doi.org/ 10.5281/zenodo.156685 |
|
DOI |
https://doi.org/10.5281/zenodo.6276508 |
|
persistent identifier |
https://treatment.plazi.org/id/03B1AA0B-FD05-2464-B612-25FC798EFDF7 |
|
treatment provided by |
Plazi |
|
scientific name |
Microlympia echina |
| status |
sp. nov. |
Microlympia echina View in CoL , new species
Figs. 116 View FIGURES 1 2 View FIGURES 3 11 View FIGURES 12 16
Types: Male holotype (FMNH), 16 additional male and 16 female paratypes (AMNH, CAS) from leaf litter in a red alder riparian forest on Alder Creek, 1.5 miles north of Hoh River, Jefferson Co, Washington, USA (47°49’43”N, 124°13’30”W), collected 28 March 2003 by W. Leonard; 3 male and 3 female paratypes collected at the same site, 1 March 2003.
Etymology: Latin adjective, echina = spiny, referring to appearance of segmental setae.
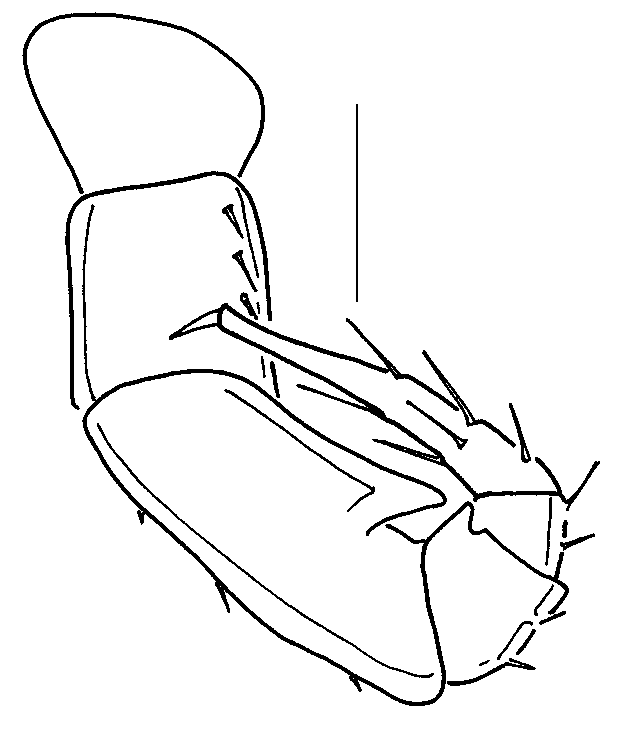
Description: Male ( Figs. 1, 2 View FIGURES 1 2 ): Twentyeight segments, two diplosegments anterior to epiproct legless. Length, 3.8 mm, width 0.35 mm. Third antennomere 5 times as long as wide, fifth antennomere 2 times as long as wide, length of sixth antennomere equal to width, sixth and seventh antennomeres subequal in length. Ocelli 8, in two rows of 4 and 3, plus single ocellus, wellformed, pigmented. Trunk segments with metaterga smooth, shining, segmental setae smooth, curving, about 1/2 width of terga, outer two setae on each side on tubercles, outermost seta on each side directed laterally, inner two setae directed medially, arching over metaterga. Legpairs 1 ( Fig. 3 View FIGURES 3 11 ) and 2 ( Fig. 4 View FIGURES 3 11 ) with tarsal combs; coxae 2 with vas deferens opening through coxa on short tube. Legpair 3 encrassate, femur with short mediodistal projection ( Fig. 5 View FIGURES 3 11 ). Legpairs 4 ( Fig. 6 View FIGURES 3 11 ) and 5 ( Fig. 7 View FIGURES 3 11 ) similar, but with stronger femoral projection. Legpair 6 ( Fig. 8 View FIGURES 3 11 ) larger than preceeding legpairs, femur with additional basal process. Legpair 7 of normal size ( Fig. 9 View FIGURES 3 11 ). Gonopods in anterior view ( Fig. 12 View FIGURES 12 16 ) with broad sternum partially fused to coxae, anterior surface of coxae slightly roughened. Anterior angiocoxites (acx, Fig. 12, 13 View FIGURES 12 16 ) broad at base, curving posteriorly, tips with small toothlike fimbriae; posterior angiocoxites (pcx, Fig. 12, 13 View FIGURES 12 16 ) sinuous, curving posteriorly. In posterior view ( Fig. 13 View FIGURES 12 16 ), anterior angiocoxites with concave tips, acute tooth subterminal; posterior angiocoxites with expanded tips; colpocoxites poorly sclerotized, irregular, saclike, with many tiny, acute scalelike teeth. Ninth legpair ( Fig. 14 View FIGURES 12 16 ) with broad, welldeveloped sternum carrying spiracles, legs reduced to single, Ushaped article. Inner arm of U with terminal gland pore, seta; outer arm with subterminal and terminal groups of 45 setae. Large, globular glands dorsal to sternum. Tenth legpair ( Fig. 15 View FIGURES 12 16 ) with coxosternum bearing gland pores; anterior margins of gland pores with 810, fine, acute, branching fimbriae, posterior margins raised as broad, thin cuticular plates. Telopodite articles ( Fig. 10 View FIGURES 3 11 ) reduced to less than 1/2 length of normal legs. Eleventh legpair with somewhat reduced telopodites ( Fig. 11 View FIGURES 3 11 ), coxae with gland openings, anteriodistal projection. Coloration white, with faint dusting of purplebrown pigment around ocelli.
Female: Length, 4.0 mm, width, 0.38 mm. Nonsexual characters as described for male, but with 5 ocelli in row of 4, plus single ocellus. Cyphopods ( Fig. 16 View FIGURES 12 16 ) with fused valves (v, Fig. 16 View FIGURES 12 16 ) and large receptacle (r, Fig. 16 View FIGURES 12 16 ) distally toothed; teeth possibly interlock with thin, vertical processes on second leg coxae (c, Fig. 16 View FIGURES 12 16 ).
Natural History Observations: All millipeds were hand collected while using an OptiVisor© 3x magnifying visor to visually search leaf litter and woody debris along an approximately 100m long section of the valley bottom of Alder Creek, a tributary of the Hoh River. The site, which has a maritime climate with very high rainfall and relatively moderate temperatures, is located approximately 21 km inland from the Pacific coastline and lies within the coastal Sitka spruce zone ( Franklin & Dyrness 1973, Henderson et al., 1989)‾commonly referred to as temperate rainforest. The valley floor of Alder Creek is vegetated by a red alder ( Alnus rubra )/sword fern ( Polystichum munitum ) community; approximately 20 m from the stream channel, the vegetation transitions to secondgrowth western hemlock ( Tsuga heterophylla ) forest. While millipeds were present in litter throughout the forest, they were noticeably most abundant beneath sword fern clumps and in mats of alder leaves abutting large logs. Both the dense thatch beneath sword ferns and large logs provide moist retreats that can buffer the effects of seasonal drying and changing temperatures, and apparently are important microhabitats for litter invertebrates in Pacific Northwest forests.
It is surprising M. echina has not been found in WL’s collections from other survey sites in western Washington. M. echina appeared to be common at the Alder Creek site, and similar riparian forest habitat is widespread across much of lowland western Washington. Moreover, a comparable maritime climate extends along the entire Pacific Northwest coast. While it appears that M. echina may have a narrow distribution, we anticipate locating additional populations on the western Olympic Peninsula and, possibly, southern Vancouver Island, British Columbia, which harbors several regionally endemic invertebrate species (Ovaska et al., 2003; Gardner & Shelley, 1989; Shelley 1994) and genera (e.g., McKeyFender et al., 1994) that also occur on the Olympic Peninsula.
Discussion: Monotypic genera and monobasic families are justified under a variety of circumstances (see Hormiga, 1994, for more detailed argumentation). Primary among these is the argument that the species or genera so designated represent isolated sistergroups of their closest relatives; this is possible where wellcorroborated phylogenetic hypotheses are available‾hardly the case in chordeumatid millipeds. Monotypy may be used to emphasize significant phenetic distance between the taxon in question and its nearest relatives, which distance may be narrowed by subsequent discoveries, resulting in the synonymy of the monotypic taxa (for examples, see Shear, 1972, 1990), or they may reflect the confidence of their describers that they will, with future work, “fill up” with additional species and genera. This may take a while. Loomis named the family Apterouridae in 1966; a second species in the family has only just been discovered (Shear, in press) and curiously, nearly simultaneously, the same thing has happened for the Branneriidae ( Shear, 2003) monotypic since 1896. In any case, it can be argued that monotypic higher taxa are at least temoporarily useful, though they contain phenetic, not phylogenetic, information. The family Microlympiidae is described here because while an obvious brannerioid, the new species cannot be placed in any of the named families. The current state of our knowledge of the brannerioid millipeds is manifestly incomplete, as new taxa are continually being discovered and described. The brannerioids are all very small millipeds, most of the species less than 5 mm in length, are inhabitants of the poorly collected forest leaflitter habitat, and probably are for the most part winteractive. These factors have ensured that the growth of our understanding of the superfamily has been slow, and will probably continue to be so. Under the circumstances, the discovery of a new form that differs very significantly from those already known is best recognized by establishing a higher monotypic/monobasic taxon, which becomes a “red flag” encouraging systematists to look for additional, related species.
The form of the ninth and tenth legs in Microlympia echina is unique, providing two sound autapmorphies for the family. The pregonopodal leg modifications are not found elsewhere in Brannerioidea.
Comparisons with possible candidate families further emphasize the distant position of Microlympia echina . The family Tingupidae Loomis 1966 was originally monobasic but now contains three genera ( Tingupa Chamberlin 1910 [ten species], Buotus Chamberlin 1940 [monotypic], and Blancosoma Shear and Hubbard 1998 [monotypic]). The gonopods of Tingupa were thoroughly studied by WS ( Shear, 1981) and follow the typical brannerioid plan, with twopart angiocoxites and a lobelike or saclike colpocoxite. The threebranched posterior angiocoxite is remarkably uniform in the genus, while the anterior angiocoxite is divided on each side into median and lateral plates. The ninth legs retain 2 or 3 telopodite articles and the tenth legs are unmodified. Species of Tingupa are heavily sclerotized, have prominent metatergal paranota and a metatergal sculpture of short, thin, acute ridges ( Shear, 1981, Figs. 1, 2 View FIGURES 1 2 ). The segmental setae are short and spatulate. The unique ninth legpair and modified tenth legpair of M. echina , the lack of paranota and segmental sculpture, weak sclerotization and long, acute segmental setae all point to considerable differences from tingupids. The monobasic Japanese family Niponiosomatidae Verhoeff 1941 ( Shear, 1988) is worth considering also, because of its close relationship to the Tingupidae and the many biogeographical connections between Japan and northwestern North America. The same arguments regarding the gonopod complex of tingupids vs. Microlympia can be made for this family. The Branneriidae Cook 1896 ( Shear, 1972, 2003) consists of two species of Branneria from southeastern North America; the gonopods differ strongly from those of the tingupids, niponiosomatids and Microlympia in the presence of a pseudoflagellar branch. However, both the ninth and tenth legpairs are strongly reduced, the tenth much more so than in Microlympia , and the glands of the eleventh coxae may be vestigial. In addition, several taxa of small, obscure European chordeumatidans have been placed in Brannerioidea ( Shear, 2000). The families Trachygonidae Cook 1896 , Heterolatzeliidae Verhoeff 1897 , and Chaemosomatidae Verhoeff 1913 remain poorly studied, and each contains one or a few species. Closer looks at these taxa may result in future synonymies in the Brannerioidea.
Some features of Microlympia echina deserve further comment. A trend toward reduction of segment number from the ground plan of 32 ( Shear, 2000) often accompanies small size in chordeumatidan millipeds. The present minimum known number is 26 segments, found in both sexes of, for example, Branneria carinata (Bollman) ; many species have males with 28 segments and females with 30, while others have 28 segments in both sexes, as does M. echina . The legless condition of the two segments anterior to the epiproct in the present species suggests the possible future disappearance of those two segments altogether, giving 26segmented males and females.
The absence in this species of the expected promentum, thought to be present in all members of the Suborder Craspedosomatidea ( Shear, 2000) may be due to small size, but a discernable promentum is present in some tingupids of similar stature. It may be necessary in the future to reevaluate the importance of this character.
Having evidently functional coxal glands on the ninth legpair is a plesiomorphic character. In more apomorphic forms, the glands are either vestigial (as in many trichopetalids), completely absent, or permanently extruded and sclerotized as colpocoxites (characteristic of the superfamily Heterochordeumatidea; Shear, 2000). In M. echina , this plesiomorphic character is correlated with the highly apomorphic strong reduction of the legs themselves. The homologies of the remains of the reduced legs are phylogenetically important, but hard to establish in this species. One hypothesis is based on the presence of two groups of setae on the single articlesthe basal group may represent the distal limits of the coxa, while the apical group may mark an extremely reduced telopodite. In other brannerioids, telopodite reduction takes place as a result of the loss of podomeres, not podomere fusion, so the telopodite remnant is likely to be the last vestige of the prefemur, fused to the reduced coxa. However, the question can only be answered by the examination of antepenultimate and penultimate males, none of which were collected.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.