Orthochromis katumbii, Schedel & Vreven & Manda & Abwe & Manda & Schliewen, 2018
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4461.3.1 |
|
publication LSID |
lsid:zoobank.org:pub:B646DF09-BADE-43F1-9CE5-39EF1E2971A8 |
|
DOI |
https://doi.org/10.5281/zenodo.5995497 |
|
persistent identifier |
https://treatment.plazi.org/id/67302D41-BE98-412B-8F93-F3FE44A50505 |
|
taxon LSID |
lsid:zoobank.org:act:67302D41-BE98-412B-8F93-F3FE44A50505 |
|
treatment provided by |
Plazi |
|
scientific name |
Orthochromis katumbii |
| status |
sp. nov. |
Orthochromis katumbii sp. nov.
Orthochromis sp. “Mambilima“— Schedel et al. 2014
Holotype. MRAC 2015-009 View Materials -P-0006 (1, 85.9 mm SL), Democratic Republic of the Congo, Kiswishi River, near confluence with Matete stream , Luapula basin (-11.486528/ 27.650306)
Paratypes. MRAC 2015-009 View Materials -P-0001 (1, 53.2 mm SL), Democratic Republic of the Congo, Kiswishi River, Futuka farm, Luapula basin (-11.488028/27.645833). — ZSM 46844 (1, ex MRAC 2015-009 View Materials -P-0002, 81.8 mm SL), Democratic Republic of the Congo, Kiswishi River, Futuka farm, Luapula basin (-11.488028/ 27.645833). — MRAC 2015-009 View Materials -P-0003 (1, 56.6 mm SL), Democratic Republic of the Congo, Kiswishi River, Futuka farm, Luapula basin (-11.488028/27.645833). — MRAC 2015-009 View Materials -P-0007-0009 (3, 58.7–85.2 mm SL), collected with holotype .— ZSM 41450 (6, 27.2–57.4 mm SL), Zambia, Luapula River below Mambilima Falls (-10.5689/ 28.6783).
Additional material. ZSM 42322 (2, 71.3–88.9 mm SL), Zambia, Luapula River below Mambilima Falls ; kept in aquarium (-10.5689/28.6783).
Differential diagnosis. Orthochromis katumbii is distinguished from all Malagarasi- Orthochromis species including O. sp. “Igamba” except O. mazimeroensis and O. rubrolabialis by having more scale rows on cheek (1–4 vs. 0). Further it is distinguished from O. kasuluensis , O. mosoensis , and O. rugufuensis by having more scales in lower lateral line (10–13 vs. 7–9) and furthermore from O. kasuluensis by having fewer dorsal-fin rays (7–9 vs. 10); from O. mosoensis by having more scales on operculum (2–3 vs. 0–1); from O. uvinzae by having fewer scales between upper lateral line and dorsal-fin origin (4–5 vs. 6-8), by having fewer dorsal-fin spines (16–18 vs. 19–20) and it is distinguished in position of pterygiophore supporting last dorsal-fin spine (vertebral count: 15–17 vs. 18- 19). From O. mazimeroensis it is distinguished by having more horizontal line scales (30–31 vs. 26–28), more abdominal vertebrae (14–15 vs. 12–13) and more total vertebrae (30–31 vs. 26–28). It is distinguished from O. rubrolabialis by having more ceratobranchial gill rakers (7–9 vs. 5–6) and total gill raker (10–13 vs. 8-9); from O. stormsi by having more caudal vertebrae (16–17 vs. 14–15), more total vertebrae (30–31 vs. 28–29), more horizontal line scales (30–31 vs. 26–28) and fewer scales between upper lateral line and dorsal-fin origin (4–5 vs. 6–9); from O. polyacanthus by having more series of scales on cheek (1–4 vs. 0); from O. torrenticola by having fewer anal-fin spines (3 vs. 4). Meristic values of O. katumbii overlap with those of O. kalungwishiensis but is distinguished by differences in colour and melanin patterns (e.g. nostril stripe in O. katumbii not extending to interorbital stripe vs. extending in O. kalungwishiensis ; operculum yellowish-grey in O. katumbii vs. reddishbrownish in O. kalungwishiensis ; vertical bars crossing midlateral band more pronounced in O. kalungwishiensis ). Meristic values of O. katumbii overlap with those of O. luongoensis but is distinguished by ratio length/depth of caudal peduncle (1.6–1.9 vs. 2.0–2.4); in addition O. katumbii tends to have fewer vertical bars on flank (7–9 vs. 9– 12). Meristic values of O. katumbii overlap with those of O. machadoi but is distinguished by smaller body depth (22.4–27.7 vs. 30.0–32.2 % SL). It is distinguished from S. neodon by having more circumpeduncular scales (16 vs. 12), and fewer dorsal-fin rays (9–10 vs. 11–12). It differs from H. snoeksi by having more scales on lower lateral line (10–13 vs. 9), more abdominal vertebrae (14–15 vs. 13), fewer caudal vertebrae (16 vs. 17), more analfin rays (7–9 vs. 5–6) and more total gill rakers (10–13 vs. 9), in position pterygiophore supporting last anal-fin spine (vertebral count: 15–16 vs. 13) and by having hypurals 3 and 4 fused (vs. clearly separated or fused with distinctly visible seam); differs from H. bakongo and H. moeruensis by having more horizontal line scales (30–31 vs. 26–28), more caudal vertebrae (16–17 vs. 12–15) and more total vertebrae (30–31 vs. 26–29). Additionally, O. katumbii differs from H. bakongo by having more dorsal fin spines (16–18 vs. 14–15), by having hypurals 1 and 2 and hypurals 3 and 4 fused (vs. clearly separated or fused with distinctly visible seam) and by position of pterygiophore supporting last dorsal-fin spine (vertebral count: 15–17 vs. 13–14) and from H. moeruensis by having more scales on upper lateral line (21–24 vs. 19–20). It differs from H. vanheusdeni by having more horizontal line scales (30–31 vs. 26–29). It is distinguished from herein newly described species O. kimpala by having more horizontal line scales (30–31 vs. 27–29), fewer scales between upper lateral line and dorsal-fin origin (4–5 vs. 6–7); from O. indermauri by having more horizontal line scales (30–31 vs. 25–29), caudal vertebrae (16– 17 vs. 14–15), total vertebrae (30–31 vs. 28–29) and by having hypurals 1 and 2 fused vs. clearly separated or fused with distinctly visible seam). Meristic values of O. katumbii overlap with those of O. mporokoso but is distinguished by having fewer vertical bars on flank (7–9 vs. 13–15) and in head mask pattern (i.e.: no cheek stripe present vs. present in O. mporokoso ). Meristic values of O. katumbii overlap with those of O. gecki but is distinguished by having a wider interorbital (15.5–21.7 vs. 9.6–12.9 % HL), moreover O. katumbii lacks eggspots on anal fin (vs. present in O. gecki ).
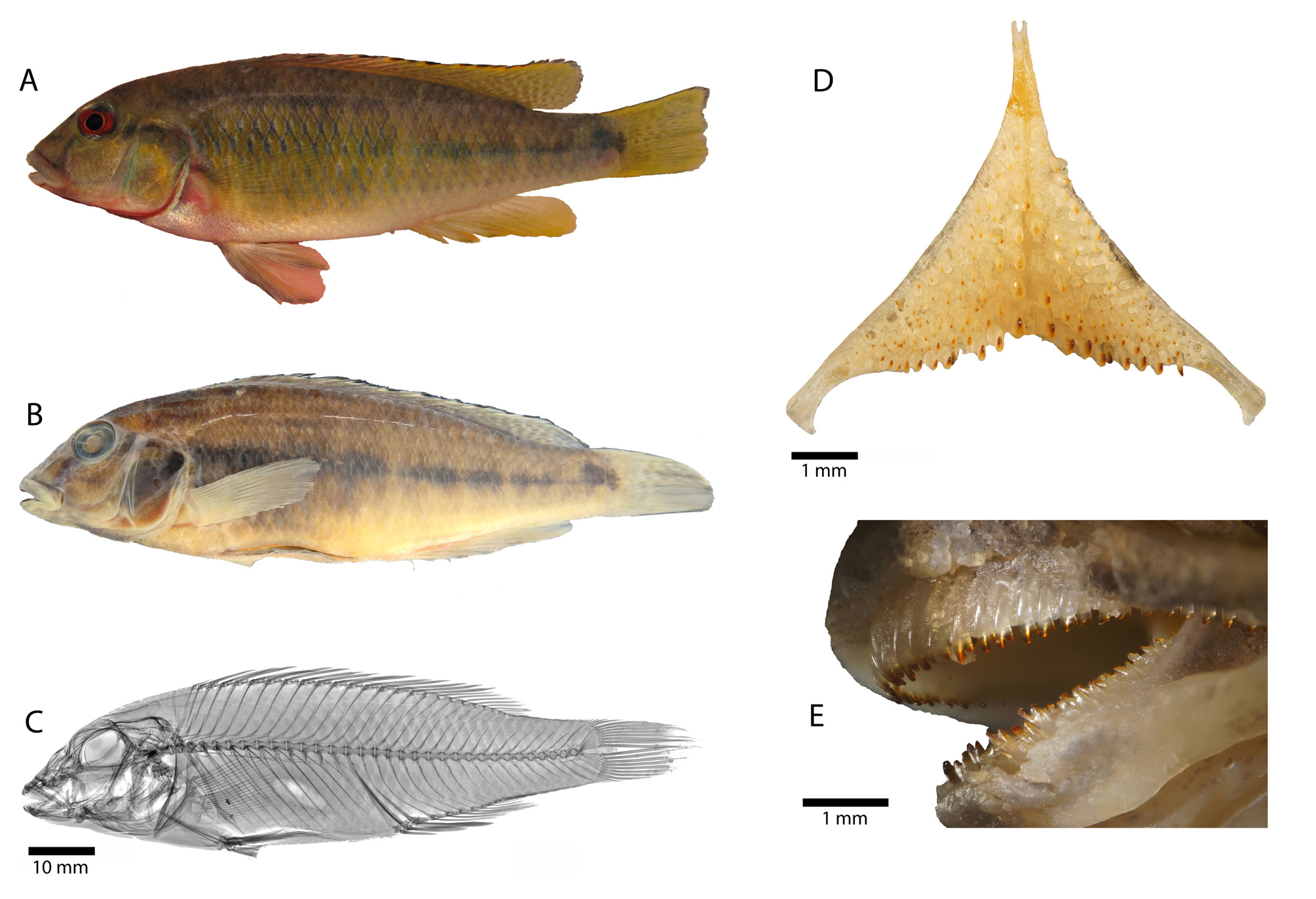
Description. Morphometric measurements and meristic characters are based on 13 type specimens. Values and their ranges are presented in Table 3. For general appearance see figure 4. Maximum length of wild caught specimens 85.9 mm SL. Moderately slender species with maximum body depth (28.1 % SL) at level of first dorsalfin spine (smaller specimens) or slightly behind dorsal-fin origin (larger specimens), decreasing towards caudal peduncle. Caudal peduncle rather elongated and moderately deep (ratio of caudal peduncle length to depth: 1.6– 1.9). Head length about one third of standard length. In adult specimens dorsal head profile gently curved and without prominent nuchal gibbosity. Dorsal head profile of subadult specimens more distinctly curved ( Fig. 9 View FIGURE 9 ). Eye diameter larger than interorbital width. Jaws isognathous or slightly retrognathous. Posterior tip of maxilla reaching vertical between nostril and anterior margin orbit. Lips not noticeably enlarged or thickened. Two separate lateral lines.
Squamation. Flank above and below lateral lines covered with comparatively large ctenoid scales. Anterior dorsal and ventral flank covered by cycloid scales. Belly with comparatively small cycloid scales. Chest covered with minute, deeply embedded cycloid scales; chest to flank transition with slightly larger cycloid scales. Snout scaleless. Interorbital scales cycloid and deeply embedded. Nape and occipital region with medium sized cycloid scales. Cheeks covered by small, partly embedded cycloid scales; 2–4 scale rows on cheek. Cycloid scales on operculum of variable size (small to medium) and shape (ovoid to circular); opercular blotch only partially covered by medium sized scales, but posterior margin always scaleless. Two to three scales on horizontal line starting from edge of postero-dorsal angle of operculum to anterior edge of operculum.
Upper lateral line scales 21–24, lower lateral line 10–13. Horizontal line scales 30–31. Caudal fin with 0–2 pored scales. Upper and lower lateral lines separated by two scales; 4–5 scales between upper lateral line and dorsal-fin origin. At level of last dorsal-fin spine one dorso-ventrally compressed cycloid scale and one normal sized ctenoid scale between origin of last dorsal-fin spine and upper lateral line. Anterior part of caudal fin covered with 3–4 vertical columns of small cycloid scales; with median scales being slightly larger; scaled area of caudal fin extended posteriorly, especially at upper and lower area, with minute, interradial scales (approximately up to two fifths of caudal fin). Sixteen scales around caudal peduncle.
......continued on the next page
Jaws and dentition. Anterior teeth of outer row of upper and lower jaw bicuspid to subequally bicuspid, large and closely set; more posterior teeth becoming subequally bicuspid, towards corner of mouth teeth smaller and less closely set and unicuspid. Individual bicuspid teeth with minimally expanded brownish crown, cusps slightly compressed and moderately widely set, neck moderately slender. Outer row of upper jaw with 29–52 teeth and outer row of lower jaw with 24–39 teeth (specimens: 37.2–85.6 mm SL). Larger specimens generally with more teeth. Two to three (rarely one or four) inner upper and lower jaw tooth rows with small tricuspid teeth. Generally larger individuals with more inner tooth rows. Lower pharyngeal bone ( Fig. 4 View FIGURE 4 ) of single dissected paratype (MRAC 2015-009-P-0007-0009, 77.2 mm SL) about 1.2 times wider than long with short anterior keel about 0.4 times length of dentigerous area. Dentigerous area of lower pharyngeal bone about 1.4 times wider than long, with 12+12 (empty tooth-sockets included) teeth along posterior margin and 6–8 (empty tooth-sockets included) teeth along midline. Anterior pharyngeal teeth (towards keel) bevelled and slender; those of posterior row larger than anterior ones, bevelled (bicuspid; well-developed major and minor cusp). Largest teeth medially situated in posterior row. Teeth along midline slightly larger than more lateral ones.
Gill rakers. Total gill raker count 10–13 with 2–4 epibranchial, one angle, and 7–9 ceratobranchial gill rakers. Most anterior ceratobranchial gill rakers smallest, increasing in size towards cartilaginous plug (angle). Anterior gill rakers on ceratobranchial unifid, towards cartilaginous plug sometimes bifid. Gill raker on cartilaginous plug shorter than longest ceratobranchial gill raker and epibranchial gill rakers further decreasing in size.
Fins. Dorsal fin with 16–18 spines and with 9–10 rays. First dorsal-fin spine always shortest. Dorsal-fin base length between 54.0–58.1 % SL. Posterior end of dorsal-fin rays almost reaching caudal-fin base; posterior tip of anal fin ending before caudal fin base. Caudal fin outline subtruncate and composed of 27–29 rays (16 principal caudal-fin rays and 11–13 procurrent caudal-fin rays). Anal fin with 3 spines (3rd spine longest) and 7–9 rays. Analfin base length between 14.8–20.2 % SL. Pectoral fin with 15 or 16 rays. Pectoral-fin length between 19.5–23.8 % SL; longest pectoral ray not reaching level of anus. First upper and lower pectoral-fin rays very short to short. Pelvic fin with 1 st spine thickly covered with skin, and 5 rays. Pelvic fin base slightly further posterior pectoral fin base. Longest pelvic-fin ray almost reaching (especially in smaller specimens) or ending well before anus (ending approximately 2 flank scales width before).
Vertebrae and caudal fin skeleton. 30–31 total vertebrae (excluding urostyle element), with 14–15 abdominal and 16–17 caudal vertebrae. Pterygiophore supporting last dorsal-fin spine is inserted between neural spines of 15th and 16h, 16th and 17th, or 17th and 18th vertebra (counted from anterior to posterior). Pterygiophore supporting last anal-fin spine is inserted between haemal spines of 15th and 16 th vertebra or 16th and 17th vertebra. Single predorsal bone (=supraneural) present. Hypurals 1 and 2 as well as hypurals 3 and 4 always fused.
Colouration in life (based on field photographs of adult specimens). ( Fig. 4 View FIGURE 4 ) Body ground colouration pale brown to yellowish. Dark grey to brownish, interrupted midlateral band extending from operculum to just behind caudal fin base ending as a blotch (less distinct than in O. luongoensis and sometimes hardly visible at all); midlateral band intensity varies depending on mood, sometimes fainting to greyish band. Midlateral band crossed by 7–10 light brown to sooty black vertical bars; these bars are short (extending shortly above and below midlateral band) and rather faint in colouration and not always recognizable. However, it should be mentioned that intensity of body markings is strongly dependent on motivational state. Chest light beige with some reddish sparkles (especially in bigger specimens). Belly light beige. Dorsal head surface and snout pale brown to greyish; cheek beige to yellow-greyish. Iris reddish at level of interorbital stripe/anterior extension of midlateral band (red more prominent in bigger specimens). Lower jaw and mental area pale beige to reddish. Throat and branchiostegal membrane reddish (ventral side of branchiostegal membrane in O. luongoensis blackish). Operculum beige to yellow-greyish with a dark grey to blackish opercular spot connecting anterior extension of midlateral band that ends almost at posterior edge of eye. Another light brownish element of variable form and intensity on ventral corner of operculum; such element also present in O. luongoensis but less intense in H. katumbii . Dark grey to brownish lachrymal stripe ending at posterior end of upper lip. Thin, dark grey to brownish nostril stripe (sometimes interrupted) in form of flattened U extending between nostrils. Dark grey to brownish interorbital stripe more intense than nostril stripe. No supraorbital stripe present. Upper and lower lip beige to pale brown, lower margin of upper lip greyish, lower lip lighter then upper lip. Dorsal fin membrane light orange to pale brown with columns of light reddish-orange to brownish maculae between branched rays and to some degree between last dorsal-fin spine (membrane between maculae brighter, almost hyaline); spinous dorsal fin with black marginal band and reddish-orange lappets; marginal band extending to some degree onto rayed part of dorsal fin. Anal fin light orange to pale brown, more intensively coloured towards distal margin. Spinous anal fin with faint reddishorange margin. No maculae or eggspots present. Caudal fin light orange to pale brown becoming more intensively coloured near margin; membrane between rays with three vertical columns of small greyish maculae (membrane between maculae brighter, almost hyaline, especially in central part of caudal fin). Outer caudal-fin rays with dark orange to blackish margin. Pectoral fin light orange, especially rays of this colour. Pelvic fin compared to pectoral fin less coloured, appearing almost transparent, membrane of pelvic fin spine greyish.
Juvenile colouration in life. (based on photos of tank-raised juveniles approximately 25 mm SL; Fig. 9 View FIGURE 9 ) Ground colouration greyish, belly beige. Patterns and stripes of head as described for adults. Greyish vertical bars on flanks more prominent than in adults. Iris greyish. Dorsal fin hyaline with some blackish spots on membrane; all other fins hyaline.
Colouration in alcohol. Colouration and melanin patterns similar to live specimens, but due the preservation procedure of specimens, i.e., first formalin fixation, transfer to 75 % EtOH etc., specimens tend to lose original colouration (especially melanin patterns more intense than in live specimens). Overall body ground colouration brownish; dorsum, flank and caudal peduncle brownish becoming beige at ventral side (band of one to two scales ventrally of flanks and caudal peduncle). Chest beige to light brownish and belly beige. Branchiostegal membrane light greyish, ventral side of branchiostegal membrane dark brown, towards anterior tip becoming brighter. Dorsal head surface brownish as dorsum, ethmoidal area becoming greyish-brown. Upper lip light greyish to beige; lower margin of upper lip greyish; lower lip beige. Cheek beige to brownish; centrally below eye a brownish blotch of variable intensity visible (as in O. luongoensis , which is not the case in living specimens). Operculum brown to dark brownish with opercular spot as described above; light brownish element of living specimens hardly visible or indistinguishable from operculum ground colouration in conserved specimens. Markings of head mask dark brownish to dark grey. Midlateral band dark brownish and vertical bars light brownish (less distinct than midlateral band). Dorsal fin greyish with black margin, subsequently followed by beige lappets; greyish maculae mainly on rayed part still visible but less intense. Anal fin whitish to beige. Pectoral fin beige. Pelvic fin beige; membrane of spine light greyish. Caudal fin light, at base pale brownish, caudally becoming beige; greyish maculae still present but less intense; margins blackish.
Distribution and biology. Orthochromis katumbii is known from Kiswishi River, a western tributary of the Luapula and from the Mambilima Falls on the Luapula ( Fig. 1 View FIGURE 1 ). At the type, locality the Kiswishi River is about ten meters wide and on average about one meter deep and the bottom substrate consists of gravel and smaller rocks ( Fig. 8 View FIGURE 8 ). Water temperature varied between 19.3 and 23.8 °C (measured in August and September), pH between 7.73–7.95, electrical conductivity 377.7 and 380.1µS. O. katumbii is a benthic-rheophilic maternal mouthbrooder with clutch sizes, in captivity, of between 25 and 30 eggs (pers. comm. J. Geck). Recently a monogenean gill parasite Cichlidogyrus consobrini Jorissen, Pariselle and Vanhove 2017 was described from specimens obtained from O. katumbii and Sargochromis mellandi ( Boulenger 1905) .
Etymology. The species is named after Mr. Moïse Katumbi who supported part of the 2015 ichthyological research field expedition of the Mbisa Congo project in Katanga province of the DRC, who himself is a great fish enthusiast. Some specimens of the new species were collected on his farm “Ferme de Futuka”.
| ZSM |
Bavarian State Collection of Zoology |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.