Peronia persiae, Maniei & Espeland & Movahedi & Wägele, 2020
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4758.3.5 |
|
publication LSID |
lsid:zoobank.org:pub:2F2B0734-03E2-4D94-A72D-9E43A132D1DE |
|
DOI |
https://doi.org/10.5281/zenodo.3812108 |
|
persistent identifier |
https://treatment.plazi.org/id/7580B29C-1376-4776-9F91-96121A0C2502 |
|
taxon LSID |
lsid:zoobank.org:act:7580B29C-1376-4776-9F91-96121A0C2502 |
|
treatment provided by |
Plazi |
|
scientific name |
Peronia persiae |
| status |
sp. nov. |
Peronia persiae View in CoL sp. nov. Maniei, Espeland, Movahedi & Wägele
( Figs. 2–10 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 View FIGURE 7 View FIGURE 8 View FIGURE 9 View FIGURE 10 )
ZooBank LSID: urn:lsid:zoobank.org:pub:2F2B0734-03E2-4D94-A72D-9E43A132D1DE
Type material. Holotype: Zoologische Staatssammlung München ( ZSM Mol 20180017), Lavan Island (26°48′20.99″N 53°16′4.80″E), collected in February 2016, 35 mm in length GoogleMaps . Paratypes: Zoologische Staatssam- mlung München ( ZSM Mol 20180018), Bandar Lengeh (26°33′29″N 54°52′50″E), collected in March 2015 (1 specimen), 32 mm in length; Lavan Island (26°48′20.99″N 53°16′4.80″E), collected in February 2016 (2 speci- mens), 22 and 37 mm in length GoogleMaps .
Etymology. Peronia persiae sp. nov. is named after the home country of the first author, Iran ( Persia).
External morphology ( Figure 2 View FIGURE 2 ). Living specimens with 20–65 mm in length, and 13–37 mm when preserved in formalin. Elongate while moving, but more oval to round in outline when resting. Notum of living animals muddy green to grey in colour and covered with tubercles (papilla) ( Figure 2A – B View FIGURE 2 ). Foot elongate, smooth and light green; covered by hyponotum ( Figure 2A View FIGURE 2 ). Eight to 16 tubercles bearing dorsal eyes in groups of two to four ( Figure 2C View FIGURE 2 ). In posterior region of the mantle, six to 16 irregularly branched branchial gills (so called gill-trees) ( Figure 2D View FIGURE 2 ). Gills expanding only when the animal is submerged. Two retractable tentacles with an apically lying eye between mantle and foot in the head region. Ventrally lying mouth surrounded by oral lips. Male genital pore between right tentacle and labial palp ( Figure 2A View FIGURE 2 ). Anus located ventrally at the posterior end of the body in the middle between mantle and foot; female opening on the right side of the anus; pneumostome posterior to the anus and opening only when the slug is out of the water.
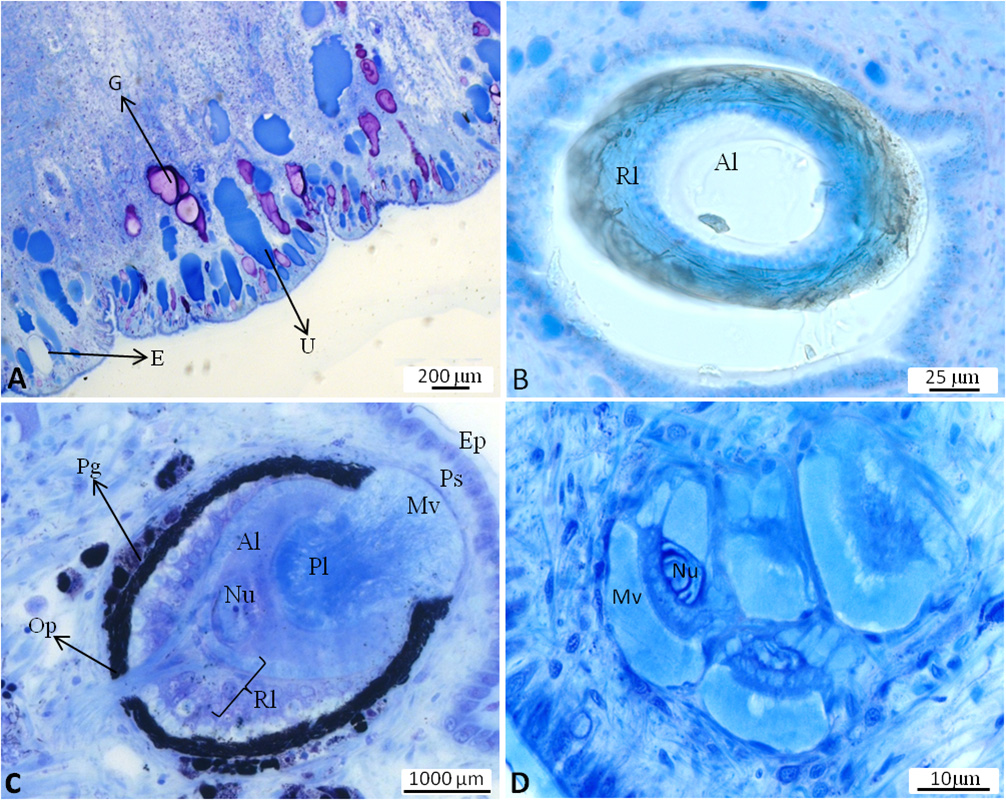
Integument. Epidermis composed of small cells; completely covered by a thin cuticular-like layer. Many bluish stained glandular cells present, reaching deep into the notum tissue; glandular cells staining violet (mucopolysaccharides) interspersed. Some cells without distinct contents (probably empty gland cells), also reaching into notum tissue ( Figure 3A View FIGURE 3 ).
Vision system. Oval shaped stalk eye on the tip of each tentacle; consisting of cornea, lens and pigmented retina layer ( Figure 3B View FIGURE 3 ). The latter comprising a villous, pigmented layer, a somatic layer and neural layer with nerve fibres. The dorsal eyes on the notum of inverse nature: the position of the retinal layer inverted with the main nerve penetrating the pigment layer surrounding the retina. Lens composed of two parts: Principal lens consisting of one cell with a large nucleus and thick microvilli layer facing towards epidermis. Accessory lens lying beneath principle lens; formed by at least one cell with a large nucleus. Epidermis covering dorsal eyes similar to surrounding epidermis, but with smaller cells above the eyes ( Figure 3C View FIGURE 3 ). Photoreceptors as extraocular structures scattered beneath the epidermis, partly single or forming clusters of up to eight receptors and sometimes in close vicinity to dorsal eyes. Each receptor composed of a cell with a thick layer of microvilli and large nucleus. No retina or cornea observed. Photoreceptors separated by connective tissues and probably muscle cells ( Figure 3D View FIGURE 3 ).
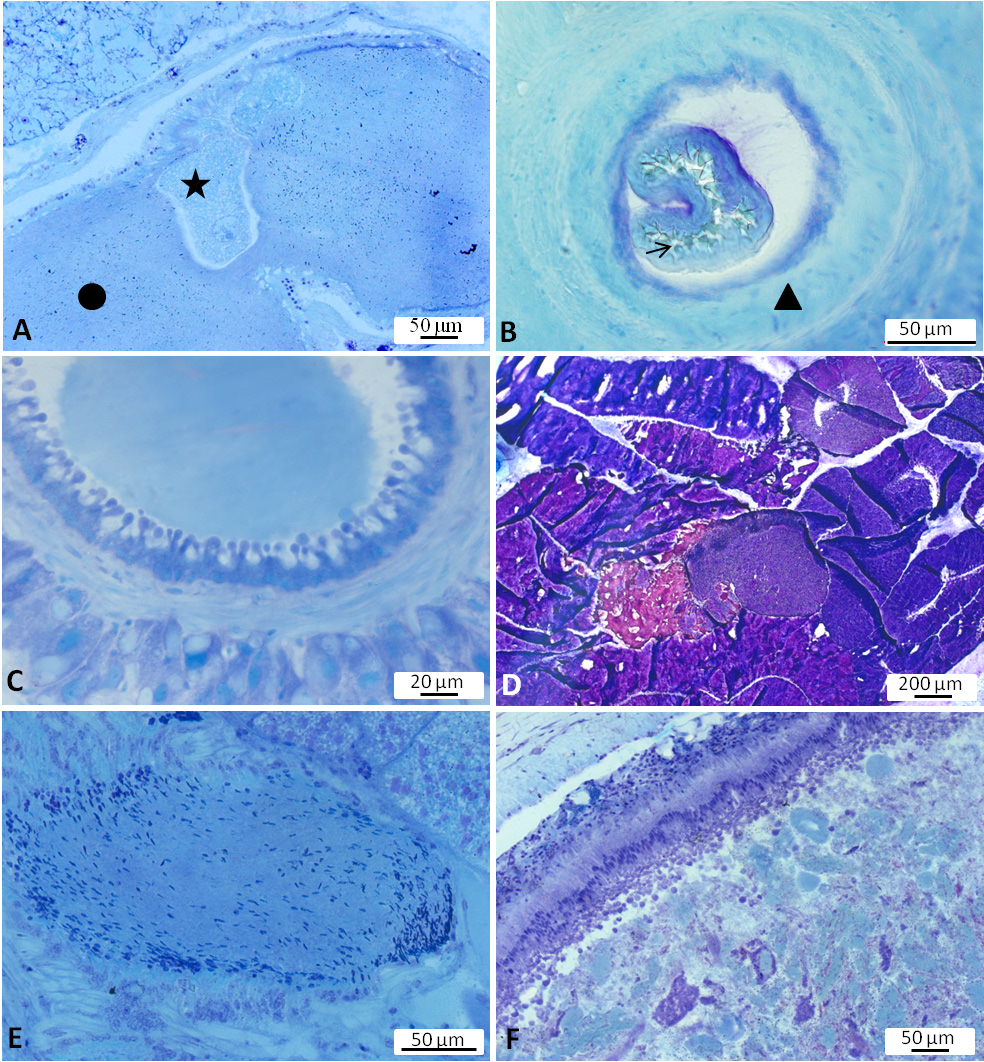
Digestive system. Radula broad, with teeth lying on a thick cuticular membrane ( Figure 4A View FIGURE 4 ). Interior of odontophore filled with red staining substance reminding of hyaline material or connective tissue (“elastic non-cellular substance”, Awati & Karandikar 1948, p. 14). Odontophore flanked by the lateral cartilage-like radula cushions as well as pharyngeal muscles; radula cushions connected by a rigid area probably consisting of red stained connective tissue ( Figure 4A View FIGURE 4 ). Radular formulae counted for 11 specimens; formulae ranging from 49 × 47.1.47 (specimen length alive 22mm) up to 71 × 87.1.87 (specimen length alive 65 mm). Rachidian teeth tricuspid with a main middle cusp and one distinctly narrow and long lateral cusp on both sides of main cusp ( Figure 5A View FIGURE 5 ). Height of rachidian teeth about 50 µm. First inner lateral teeth smaller than other laterals ( Figure 5B View FIGURE 5 ); height of unicuspid lateral teeth gradually increasing from about 60 µm to 75 µm and decreasing again towards the lateral rim. Lateral teeth with a conical shape seen from lateral view and inner side curved in a concave way ( Figure 5C View FIGURE 5 ). Tips (seen from above) usually broadened and with a blunt, spatulate apex ( Figure 5 View FIGURE 5 A–B). Salivary glands on both sides of the oesophagus with small ducts leading into pharynx close to the transition into the oesophagus; composed of many finger-like tubes combining into clusters and forming a grape-like structure. Glandular cells with reddish to violet staining granular contents ( Figure 4B View FIGURE 4 ). Oesophagus with highly folded epithelium composed of ciliated columnar cells, covered in many areas with a thin homogenously staining (probably cuticular) layer—sometimes more greenish, sometimes bluish ( Figure 4C View FIGURE 4 ). Oesophageal folds underlain with red stained connective tissue and surrounded first by a longitudinal and then by a circular muscular layer. Oesophagus entering the first part of the stomach. Stomach consisting of four parts. First part characterized by a thin epithelium; receiving the ducts of the dorsal and left lateral lobes of the digestive gland. Second chamber strongly muscular ( Figure 4D View FIGURE 4 ), swollen and stratified; receiving the duct of the posterior lobe of the digestive gland. Third chamber funnel-shaped; pigmented on the outer side; its epithelium forming a highly folded structure with dendritic branches filling nearly completely the internal lumen ( Figure 4E View FIGURE 4 ). The last chamber representing a small widened section at the beginning of intestine with only thin ridges internally and ciliated cells in the epithelium. Intestine forming two distinct long loops lying close together ( Figure 6 View FIGURE 6 A–C), thus fitting best type II according to the definition of Labbé (1934). Epithelium with light blue stained cells and with violet stained goblet cells ( Figure 4F View FIGURE 4 ). Digestive gland composed of many lobes; epithelium exhibiting cells which excrete substances stained in various shades of violet to blue ( Figure 4G View FIGURE 4 ).
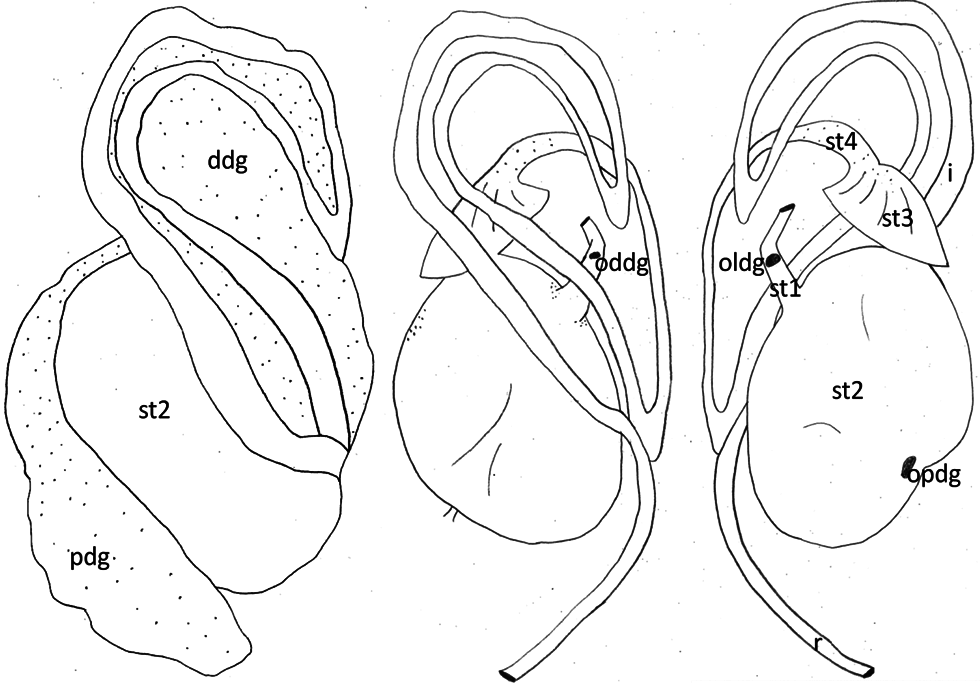
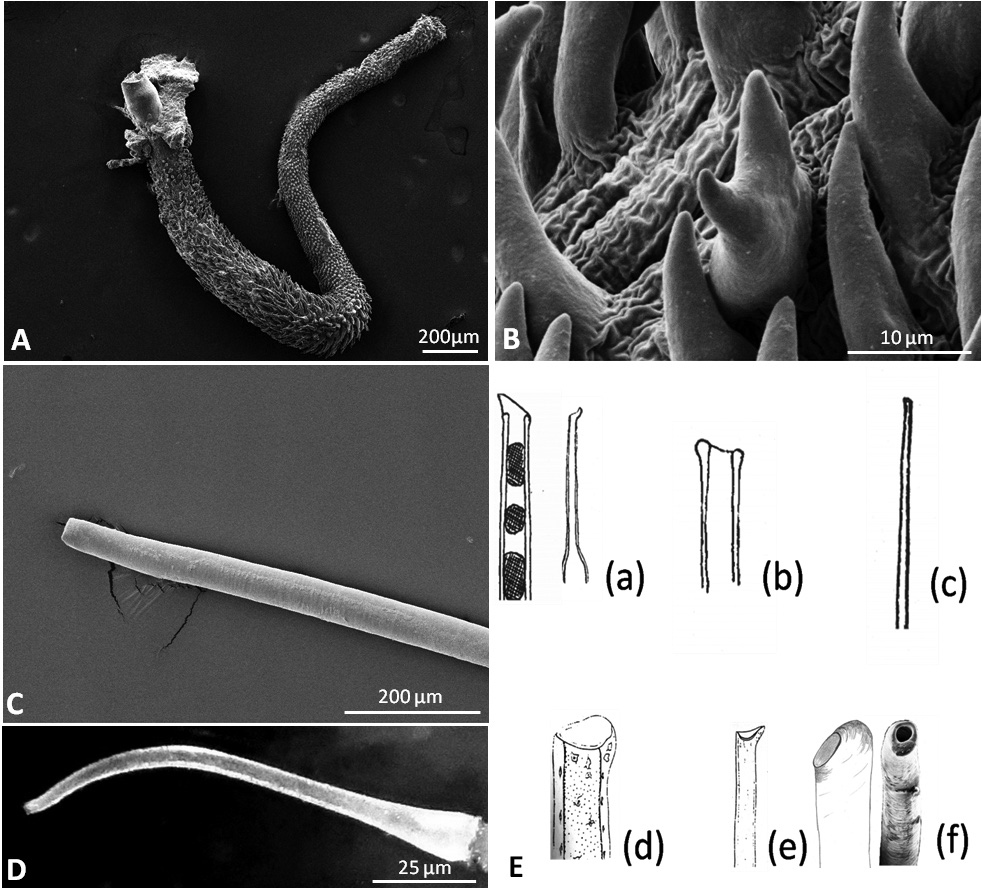
Reproductive system. Hermaphrodite gland (gonad) compact, whitish with bright dots representing the oogonia; located slightly on the left side of the visceral cavity, next to the diaphragm. Hermaphroditic duct originating from gonad and continuing to a widened area, possibly the ampulla, containing sperm, as well as a few oocytes; duct with small epithelial cells ( Figure 7A View FIGURE 7 , Figure 9A View FIGURE 9 ). Vas deferens forming a long tube starting from hermaphroditic duct in the posterior third of body, running to the right side of the head, and ending in the penis; surrounded with a thick muscle layer and lined internally by ciliated cells. Prostate gland not observed. The anterior organs of male system consisting of the penial structure (penis with vas deferens and attached retractor muscle ending in penial sac) and penial accessory gland (glandular duct, hollow needle and end sac of penial gland). Both complexes opening into a common vestibule and sharing same male opening ( Figure 9B View FIGURE 9 ). Penis usually inverted into the vas deferens ( Figure 7B View FIGURE 7 ) ending in the penial sac. The outer epidermis of penis covered with spines. During evagination, the usually outer layer turns to the outer side ( Figure 8A View FIGURE 8 ). The hooks at the apical and distal areas are short and conical while the rest of spines in the middle part are long, sharp and curved downward at the tips. Fork-shaped spines existing in different areas of the penis ( Figure 8B View FIGURE 8 ). Retractor muscle attached to the base of the penis in front part, running far into the posterior part of body. Penial accessory gland duct long, heavily coiled and swollen in posterior part, close to the opening; epithelium composed of columnar cells with apocrine secretion ( Figure 7C View FIGURE 7 ). Light yellow needle apparatus straight, narrow and hollow; in one case tip slightly curved; length of needles around 1.3 mm ( Figure 8 View FIGURE 8 C–D). The opening of needle usually blunt, only in one individual of sickle shape ( Figure 8E View FIGURE 8 ). Sac-like structure without papillae. Proximal spermoviduct folded and embedded within the female gland mass. The latter composed of capsule gland (albumen gland), followed by membrane gland, leading into mucus glands ( Figure 7D View FIGURE 7 , Figure 9A View FIGURE 9 ). Membrane gland located ventrally underneath capsule gland, composed by columnar cells filled with reddish granules, mucus gland composed of columnar cells containing basal nucleus and many small, ovoid, violet staining granules. Presence of spiral glands could not be verified. Whitish receptaculum seminis composed of small epithelial cells, with attached sperm heads (allosperms) ( Figure 7E View FIGURE 7 ). Brownish round to elongate spermatheca (or bursa copulatrix in the sense of Schmekel 1971) connecting to short distal oviduct; greenish staining contents present inside spermatheca; epithelium composed of apocrine secreting cells ( Figure 7F View FIGURE 7 ). Female opening near the anus slightly to the right side.
Circulatory and excretory system. Heart on the right side, divided into an anterior ventricle and posterior auricle within pericardial cavity. Nephridium posterior to the diaphragm. Extension of nephridium larger on the right side, than on the left side ( Figure 11C View FIGURE 11 ).
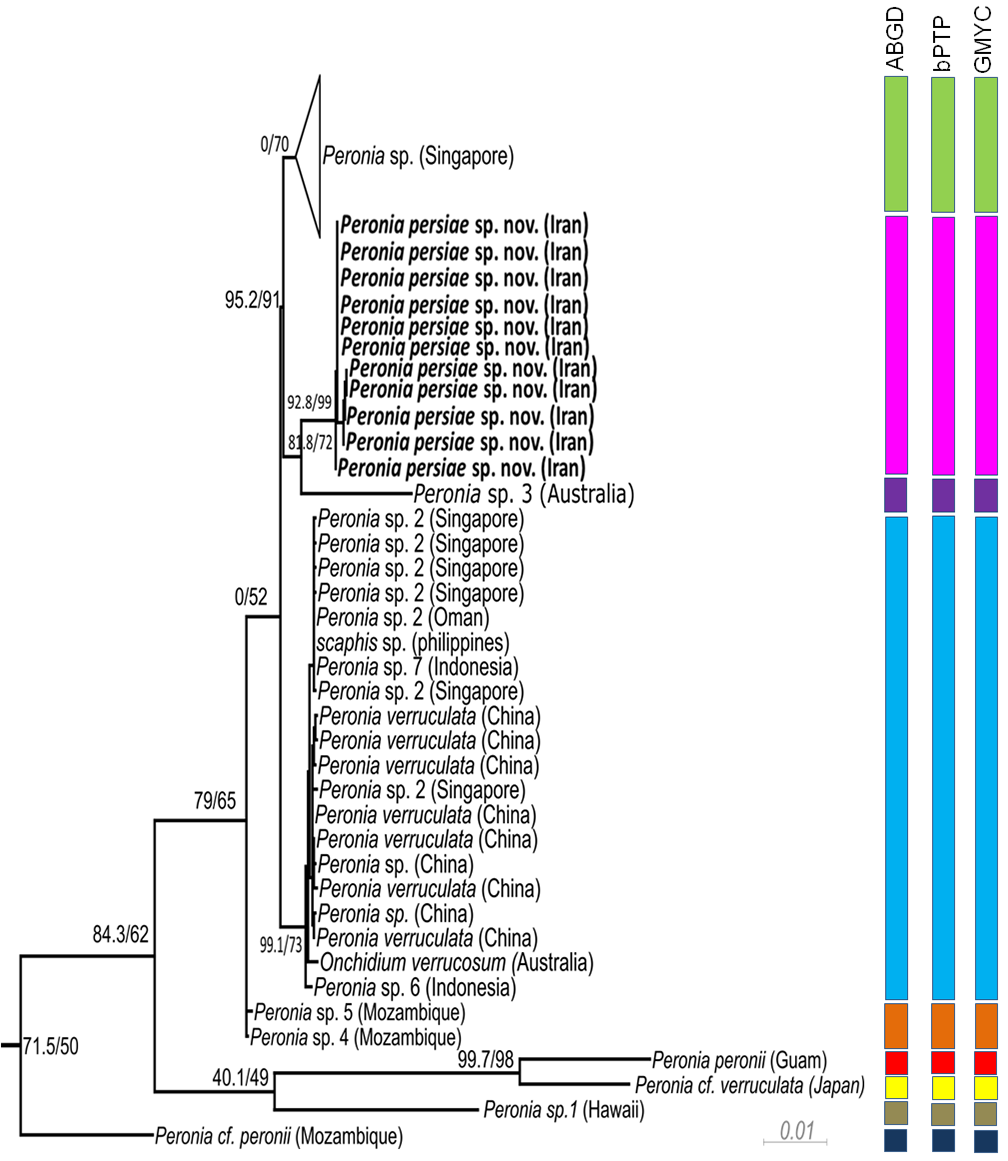
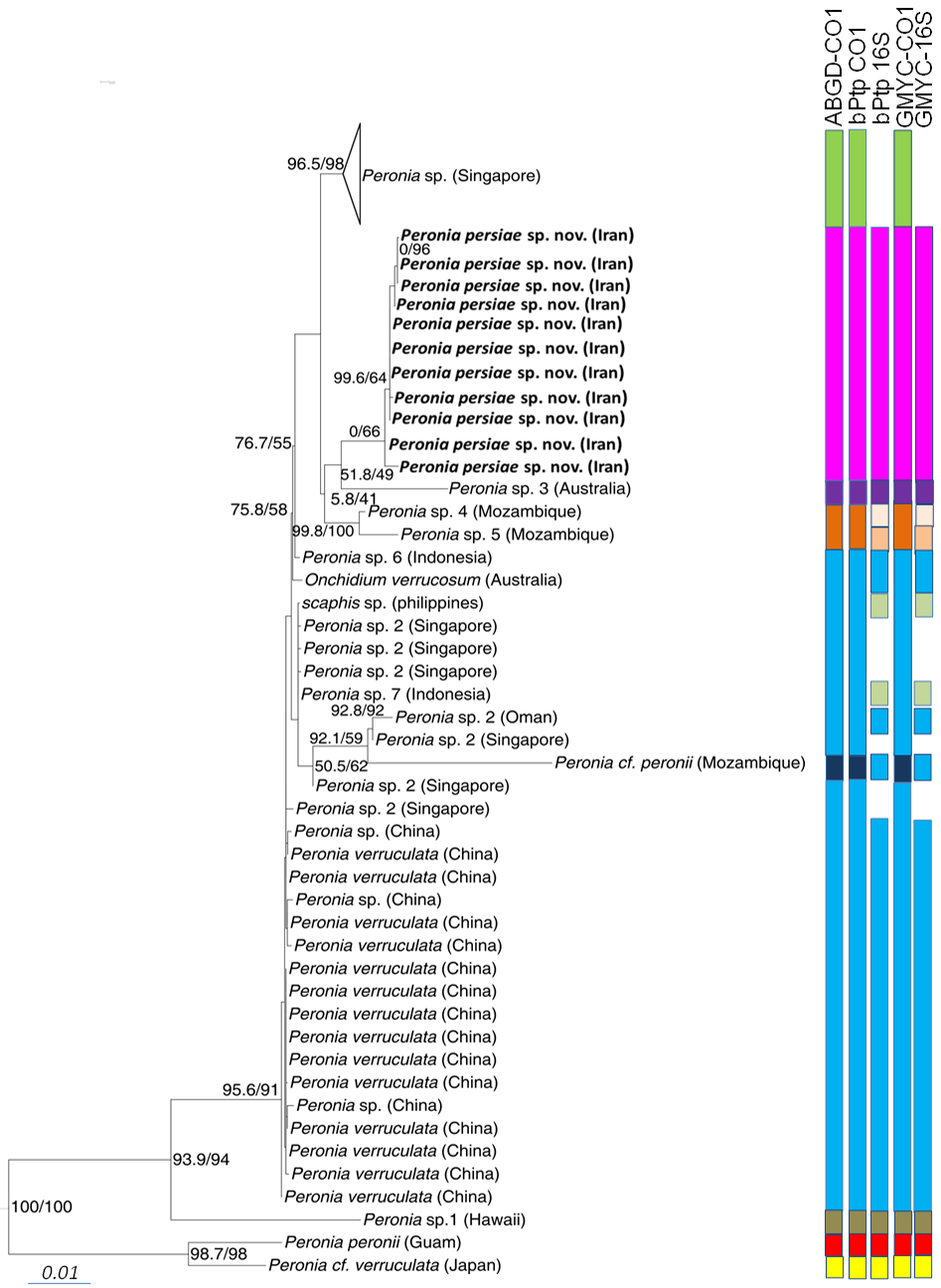
Phylogeny. The phylogenetic analyses of the concatenated dataset ( Figure 10 View FIGURE 10 ) covering 11 of the 16 acknowledged genera and including 457 sequences, provide evidence of the monophyly of Peronia with high bootstrap support in the ML analyses (99) and close relationship with a clade formed by the monophyletic Wallaconchis (96), Alionchis (100) and Paromoionchis Dayrat & Goulding, 2019 (100). The close relationship of the four genera is supported by a bootstrap value of 97. One specimen retrieved from NCBI as Scaphis sp. groups within the genus Peronia . Further monophyletic genera are Peronina (100), Melayonchis (76), Marmaronchis (100), Onchidina (100), and Onchidella (99). Monophyly of Onchidium , as shown in Dayrat et al. (2016) with a reduced dataset of Onchidiidae , cannot be seen in our tree, however misidentification of the paraphyletic genus Platevindex H. B. Baker, 1938 with members grouping with Onchidium cannot be excluded. In this overall analysis, P. persiae sp. nov. is clearly separate from all other Peronia species ( Figure 10 View FIGURE 10 ). These results are confirmed by analysing the reduced datasets including only Peronia sequences and running the analyses with COI only ( Figure 11 View FIGURE 11 , Table 3), or the concatenated alignment with COI and 16S, and analyses of distance values ( Figure 12 View FIGURE 12 ). In these two analyses, the 11 specimens of the new species always group as a separate clade, usually as sister of Peronia sp. 3, and close to Peronia sp. from Singapore (COI, Figure 11 View FIGURE 11 ) or close to Peronia sp. 4 and sp. 5 from Mozambique (concatenated data set, Figure 12 View FIGURE 12 ).
Species delimitation. All three species delimitation methods applied to the COI ( Figure 11 View FIGURE 11 ) and 16S datasets indicate nine well supported groups in the genus Peronia ( Figures 11–12 View FIGURE 11 View FIGURE 12 , S 1–S View FIGURE 1 4 View FIGURE 4 ). The Iranian specimens always form a separate taxon from P. verruculata specimens or any other Peronia species. The result of the ABGD test based on COI data shows a single and obvious barcode gap: intraspecific variability is less than 2% and interspecific variability more than 4%. The distance between the new Iranian and other close related species, Peronia sp. 4 and Peronia sp. 5, ranges between 7.0–8.0% ( Table 3). All three tests applied on the separate two genes ( Figure 12 View FIGURE 12 ) show the same results: one Singapore group, assigned to Peronia sp., is indicated as a separate species. Another group, assigned to Peronia sp. 2 from Singapore and Oman, together with sequences assigned to P. verruculata and Onchidium verrucosum (misspelling of P. verruculata ) from China and Australia, and a few sequences from differ- ent localities ( Peronia sp. from China, Peronia sp. 6 and Peronia sp. 7 from Indonesia, Scaphis sp. from Philippine Islands) are considered to be a single species. Results of GMYC and bPTP analysis, using the 16S data set, shows Peronia sp. 7 from Indonesia and Scaphis sp. as a separate group. Peronia sp. 4 and sp. 5 from Mozambique are indicated also as a single and distinct species; however, this last result was not retrieved in the GMYC and bPTP analysis using the 16S data set ( Figure 12 View FIGURE 12 ). Peronia sp. 3 from Australia and Peronia sp. 1 from Hawaii also are distinct species in all analyses. Peronia cf. peronii from Mozambique is considered as a distinct species, when applying only the CO1 dataset. This result is not confirmed in the GMYC and bPTP analysis using only the 16S data set. P. peronii from Guam (indicated as a separate species) clusters with a sequence of Peronia cf. verruculata from Japan ( Peronia sp. according to Dayrat et al. 2016), which also forms a separate species (confirmed in all analyses).
| ZSM |
Bavarian State Collection of Zoology |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |