Phlyctaenopora (Barbozia) spina, Kelly, 2021
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4990.3.13 |
|
publication LSID |
lsid:zoobank.org:pub:710F5D24-87B8-4DA2-BEE4-EE5F7BB34BAD |
|
DOI |
https://doi.org/10.5281/zenodo.5087870 |
|
persistent identifier |
https://treatment.plazi.org/id/429A66C0-04B7-496B-873B-19B935BAB894 |
|
taxon LSID |
lsid:zoobank.org:act:429A66C0-04B7-496B-873B-19B935BAB894 |
|
treatment provided by |
Plazi |
|
scientific name |
Phlyctaenopora (Barbozia) spina |
| status |
sp. nov. |
Phlyctaenopora (Barbozia) spina View in CoL sp. nov.
urn:lsid:zoobank.org:act:429A66C0-04B7-496B-873B-19B935BAB894
Fig. 1 View FIGURE 1 , Table 1 View TABLE 1
Material examined. Holotype NIWA 99630 View Materials , NIWA Stn TAN1312/D26-d45, Wanganella North (International Waters) 32.805° S, 167.467° E, 600–850 m, 9 Nov 2013. GoogleMaps
Type location & distribution. Wanganella North (International Waters), West Norfolk Ridge, 600–850 m.
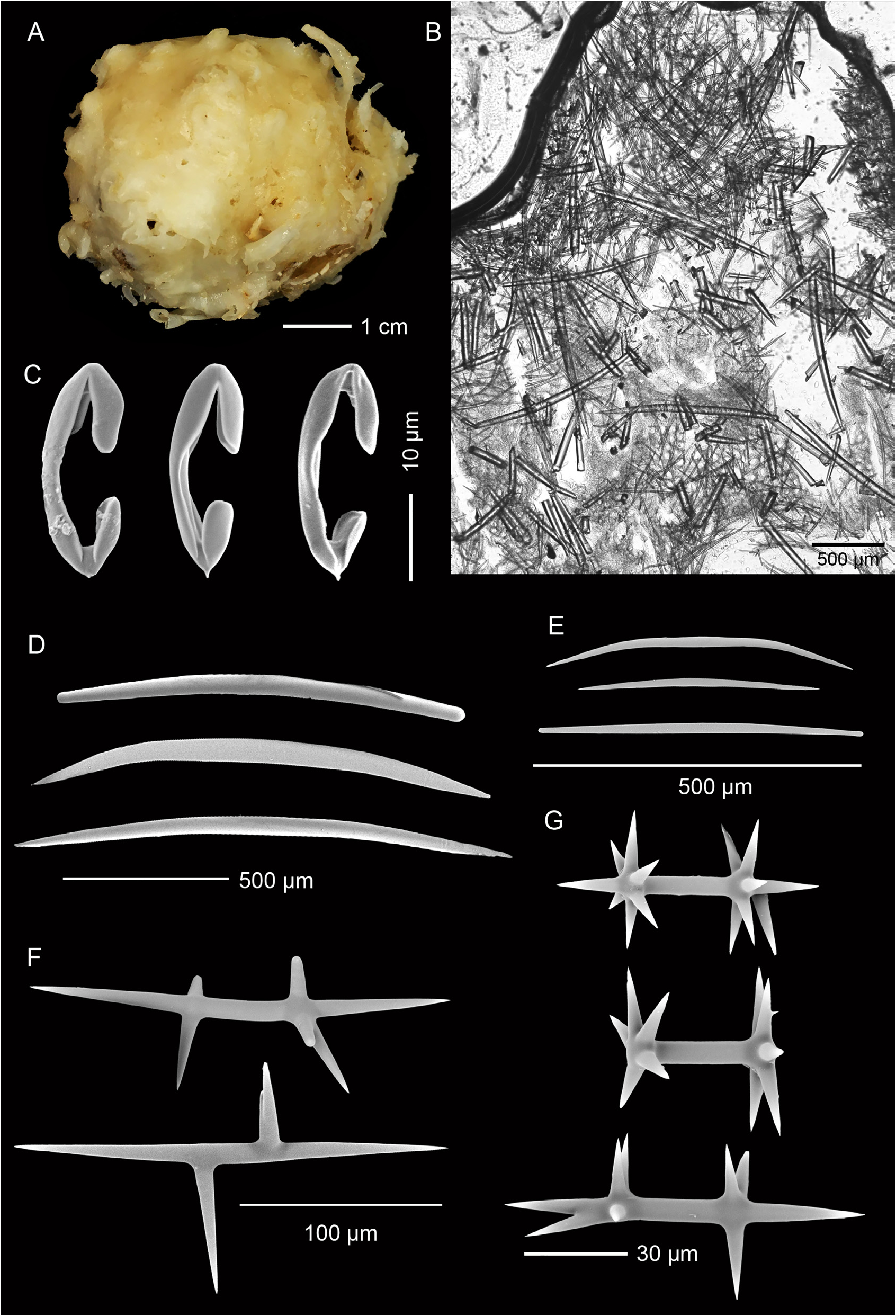
Description. Holotype ( Fig. 1A View FIGURE 1 ), hemispherical with a slightly conical apex: 30 mm high, 45 mm wide at base. A broad, compound oscule with ragged margins is situated on the apex and surrounded by numerous fistules, 8–10 mm apart, arising from a glassy smooth surface: fistules 3–7 mm long, 2–4 mm thick. Texture firm, barely compressible; fistules fragile, frequently broken. Colour in preservative, cream ( Fig. 1A View FIGURE 1 ).
Skeleton. Ectosomal skeleton a thick mass of smaller oxeas, orientated paratangentially in the outer ectosome and criss-crossed in the lower ectosome, interspersed with large oxeas; about 300 µm deep ( Fig. 1B View FIGURE 1 ). Anisochelae, abundant in outer ectosome. Larger oxeas are more-or-less perpendicular to the surface in the outer ectosome and choanosome, randomly disposed in deeper regions. Smaller oxeas abundant interstitially. Large, plesiasters-like microxeas, amphiasterlike rhabds, and anisochelae are scattered throughout the choanosome ( Fig. 1B View FIGURE 1 ).
Spicules. Megascleres ( Fig. 1D–F View FIGURE 1 ; Table 1 View TABLE 1 ) are large, thick, oxeas, smoothly curved or more commonly biangulate, with fusiform to strongylote tips; 1392 (1068–1920) µm long, 49 (35–64) µm wide, n = 20 ( Fig. 1D View FIGURE 1 ); smaller, thinner oxeas, smoothly curved to biangulate, with fusiform to strongylote tips, the latter sometimes slightly enlarged; 453 (291–662) µm long, 12 (8–17) µm wide, n = 25 ( Fig. 1E View FIGURE 1 ); large, thorned, plesiaster-like microxeas, highly modified with irregular length oxeote rays, 223 (120–360) µm long, n = 20 ( Fig. 1F View FIGURE 1 ); spicules in each category can be modified with extra rays, lumpy or spined protrusions.
Microscleres ( Fig. 1C, G View FIGURE 1 ; Table 1 View TABLE 1 ) are palmate anisochelae with a basal spur; 28 (23–32) µm long, n = 20 ( Fig. 1C View FIGURE 1 ); amphiaster-like rhabds with smooth, sharp oxeote rays; 70 (35–109) µm long, n = 20 ( Fig. 1G View FIGURE 1 ), also frequently modified.
Substrate, depth range and ecology. Dredged from rock substrate, 600–850 m.
Etymology. Named for the thorn-like form of the unique plesiaster-like microxeas ( spina , thorn; Latin).
Remarks. Phlyctaenopora (Barbozia) spina sp. nov. is differentiated from the holotype of the New Caledonian species, P. (B.) bocagei , on 1) the much larger size and form of the choanosomal oxeas [P. (B.) spina : 1425–1625 µm, mostly fusiform; P. (B.) bocagei : 1000–1250 µm, mostly strongylote]; 2) the larger size and form of the interstitial oxeas [P. (B.) spina : 453 (291–662) µm, strongylote & fusiform; P. (B.) bocagei : 300–550 µm, fusiform]; 3) the possession of larger anisochelae [P. (B.) spina : 28 (23–32) µm; P. (B.) bocagei : 18–20 µm]; 4) the possession of much larger amphiaster-like rhabds [P. (B.) spina : 70 (35–109) µm; P. (B.) bocagei : 25–45 µm]; 5) possession of a unique category of large, thorned, plesiaster-like microxeas, absent in P. (B.) bocagei ; and 6) lack of ectosomal styles present in the ectosome of P. (B.) bocagei ( Table 1 View TABLE 1 ).
In 1993, Lévi (1993) described two specimens identified as P. (B.) bocagei (MNHN DCL 3619 & 3620 in Table 1 View TABLE 1 ), that had the same general spiculation as the type specimen, but which had much larger, highly sinuous choanosomal and ectosomal strongylote oxeas, and amphiaster-like microxeas that approach the size of those in the New Zealand species. Although the ectosomal styles were not mentioned, the larger category of plesiaster-like microxeas, present in P. (B.) spina sp. nov., were absent in Levi’s 1993 specimens, justifying their retention in P. (B.) bocagei and confirming the integrity of P. (B.) spina sp. nov.
Dendy (1922) described the strongyle-like megascleres of P. (B.) primitiva as “strongylotes, usually slightly curved or bent, equi-ended, narrowing somewhat at the two ends, which are broadly rounded off.” The ends of the megascleres in P. (B.) primitiva are very similar to those of the oxeas in the New Zealand species, ranging from fusiform to strongylote, suggesting that Dendy’s ‘strongylote’ megascleres in P. (B.) primitiva are rather, a form of oxea. The similarity in morphology (hemispherical with fistules), skeletal architecture (tangential ectosome and confused choanosome), with highly modified oxea in all three species, confirms the overall integrity of subgenus Barbozia.
| NIWA |
National Institute of Water and Atmospheric Research |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |