Potamotrygon schroederi Fernandez-Yépez, 1958
|
publication ID |
https://doi.org/ 10.5281/zenodo.319945 |
|
DOI |
https://doi.org/10.5281/zenodo.5658665 |
|
persistent identifier |
https://treatment.plazi.org/id/E05B87D6-FFCA-FFB1-FF43-FAEB8E38F873 |
|
treatment provided by |
Plazi |
|
scientific name |
Potamotrygon schroederi Fernandez-Yépez, 1958 |
| status |
|
Potamotrygon schroederi Fernandez-Yépez, 1958 View in CoL
Figures 9–20 View FIGURE 9 View FIGURE 10 View FIGURE 11 View FIGURE 12 View FIGURE 13 View FIGURE 14 View FIGURE 15 View FIGURE 16 View FIGURE 17 View FIGURE 18 View FIGURE 19 View FIGURE 20 ; Tables 3–4
Potamotrygon schroederi Fernandez-Yépez (1958 View in CoL [not 1957]: 7–10; original description, Boca Apurito, Río Apure, Orinoco drainage, figured); Mago-Leccia (1970: 64; listed, Venezuela, photograph on p. 245); Castex & Yagolkowski (1970: 2–13; specimen from “Manaus”, redescription); Mago-Leccia (1978: 5; name only); Rosa (1985: 302–314; diagnosis, description, in part only); Compagno & Cook (1995: 74; compilation, from Rosa, 1985); Taphorn et al. (1997: 67; listed, Venezuela); Lasso et al. (1998: 41; holotype listed in catalog); Compagno (1999: 495; listed as valid); Machado-Allison et al. (1999: 65; listed, Venezuela); Ross & Schäfer (2000: 45–46; brief account compiled from Rosa, 1985); Carvalho et al. (2003: 25; taxonomic account); Machado-Allison et al. (2003a: 70; cited in text, Venezuela, as “ Potamotrygon schoederi ”); Machado-Allison et al. (2003b: 267; listed in table, Venezuela, as “ Potamotrygon cf. schoederi ”); Araújo (2004; conservation status, biological data, no pagination); Lasso et al. (2004: 144; listed, Venezuela, Orinoco); Compagno (2005: 541; listed as valid); Gobbo (2006: 15–16; ventral lateral line canals, figured); Rosa & Carvalho (2007: 17; listed, Brazil); Mejía-Falla et al. (2007: 126; listed, Colombia); Toffoli et al. (2008: 325-334; molecular phylogeny); Maldonado-Ocampo et al. (2008: 150; listed, Colombia); Rosa et al. (2010: 252, 260; treated as valid); Duncan et al. (2010: 17–20; exploitation, photograph, Rio Negro).
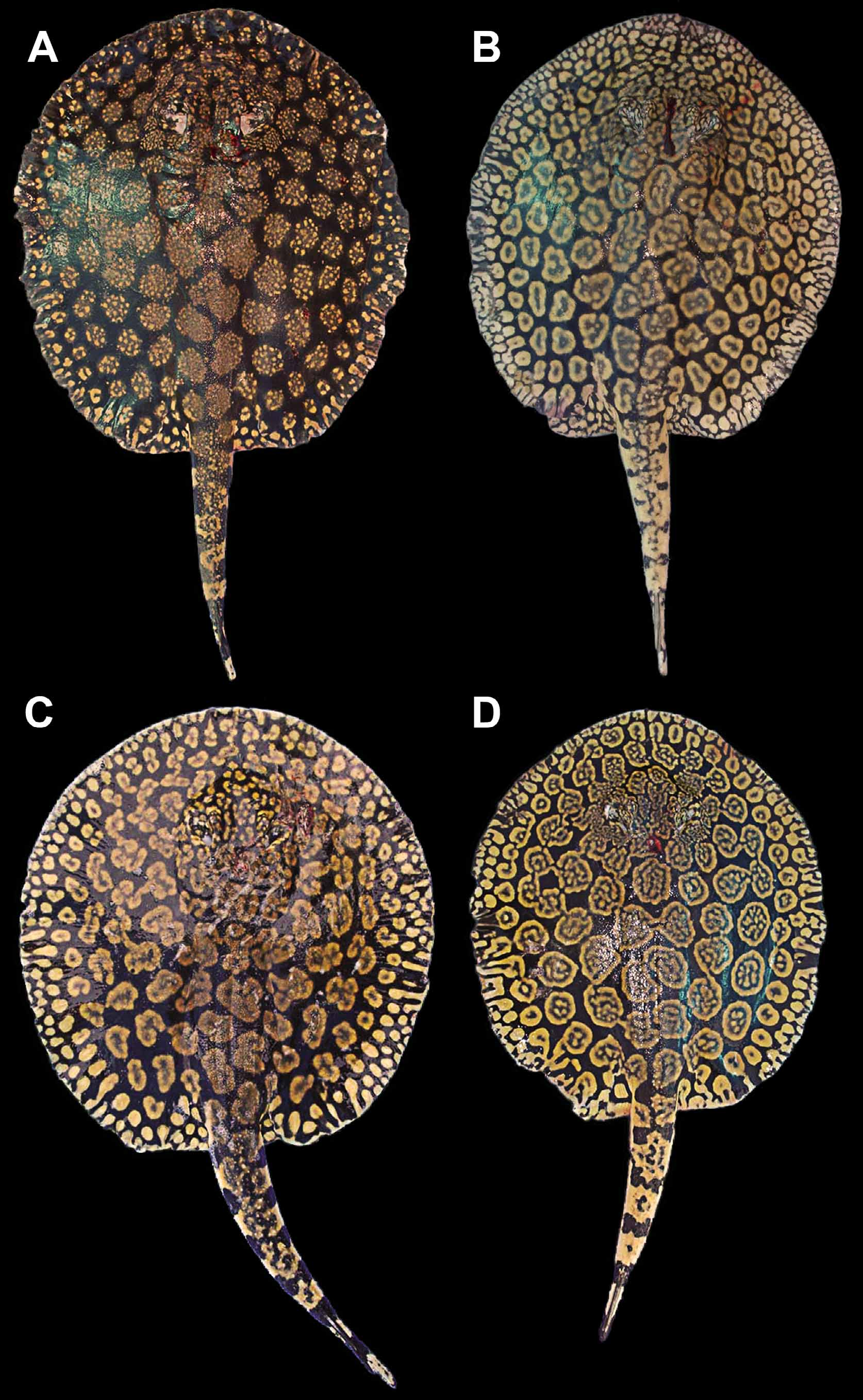
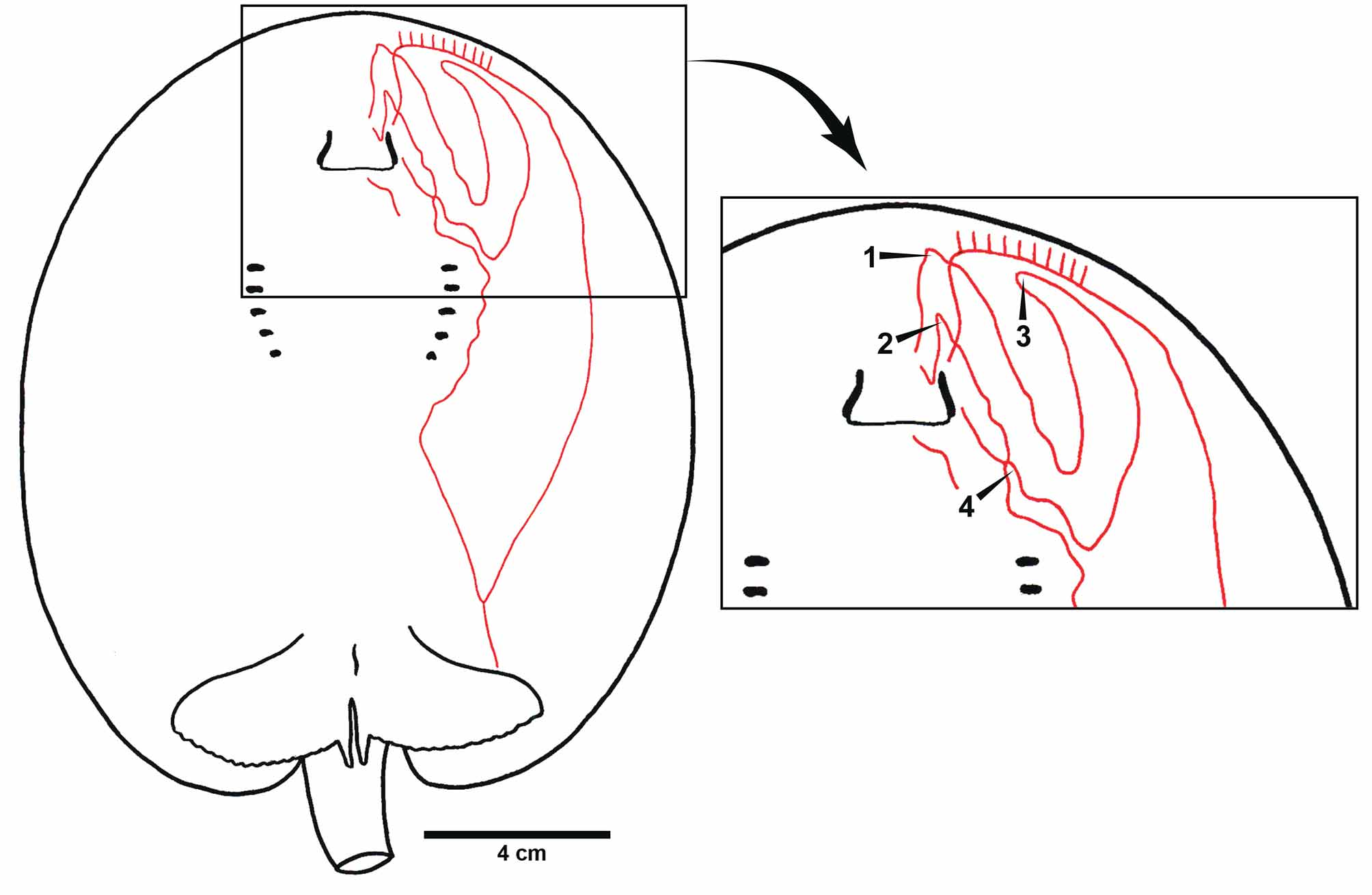
Holotype. MHNLS 2504, Río Apure, Boca Apurito, Orinoco basin, Venezuela, 16 August 1951, coll. J. Marrero (only three fragments of the original specimen remain, comprising its caudal stings and distal tail region, mouth area, and a portion of the lateral disc; Lasso et al., 1998) ( Figure 9 View FIGURE 9 ).
Other material examined. (26 specimens). Venezuela: ANSP 161193, preadult female (355 mm, 200 mm DL, 180 mm DW), Rio Caura at ferry crossing (E side) on Caicara-Ciudad Bolivar hwy., Bolivar state, Venezuela, 7° 27’N, 065°12’W, 0-2 m, 50 ft. seine, 19:00-19:45, on bottom of sand and gravel, 19 November 1985, coll. B. Chernoff, W.G. Saul, J.G. Lundberg, F. Provenzano, R. Royero (V85 -35). ANSP 191999, Río Ventuari (Orinoco basin), sandy beach and mouth of Caño Guayaje (right bank tributary), ca. 210 km northeast of San Fernando de Atabapo, Manapiare, 5°6’22.176” N, 066°12’32.976” W, 4 April 2010, M. Sabaj Pérez and T. Carvalho (sampled at night with bag seine) (VEN 10–30); AUM 44507, Río Orinoco, island W of Puerto Venado, 4.5 km S of Samariapo, 56.5 km SW of Puerto Ayacucho, 5°12’25” N, 067°48’32” W, 28 Feb 2005, coll. M. H. Sabaj, N. K. Lujan, D. C. Werneke, M. Arce et al. Brazil: MZUSP 108443, Rio Negro, Amazonas, Barcelos, 0°57’30” S, 062°56’15” W, 0 9 Jan 2011, coll. F. P. L. Marques, W. P. Duncan (RN11.14); MZUSP 108444, Rio Negro, Amazonas, Barcelos, coll. M. L. G. Araújo; MZUSP 108445, Rio Negro, Amazonas, Barcelos, 0°57’30” S, 062°56’15” W, 12 Jan 2011, coll. F. P. L. Marques, W. P. Duncan (RN11.34); MZUSP 108446, Rio Negro, Amazonas, Barcelos, coll. M. L. G. Araújo; MZUSP 108447, Rio Negro, Amazonas, Barcelos, Praia da Bananinha, Boca do Coibí, 0°39’49” S, 063°01’01” W, 14 Jan 2011, coll. F. P. L. Marques, W. P. Duncan (RN11.50); MZUSP 108448, Rio Negro, Amazonas, Barcelos, 0°57’30” S, 062°56’15” W, 16 Jan 2011, coll. F. P. L. Marques, W. P. Duncan (RN11.63); MZUSP 108449, Rio Negro, Amazonas, Barcelos, coll. M. L. G. Araújo; MZUSP 108450, Rio Negro, Amazonas, Barcelos, Lago Cunimaru, 0°49’59” S, 063°02’34” W, 12 Jan 2011, coll. F. P. L. Marques, W. P. Duncan (RN11.42); MZUSP 108451, Rio Negro, Amazonas, Barcelos, Ilha do Jacami, 0°58’48” S, 062°55’12” W, 0 6 Mar 2004, coll. F. P. L. Marques (RN04.94); MZUSP 108452, Rio Negro, Amazonas, Barcelos, coll. M. L. G. Araújo; MZUSP 108453, Rio Negro, Amazonas, Barcelos, 0°58’48” S, 062°55’12” W, 23 Jan 2005, coll. M. Domingues, N. Luchetti, M. L. G. Araújo (RN05.01); MZUSP 108454, Rio Negro, Amazonas, Barcelos, Rio Demeni, 0°44’01” S, 062°37’38” W, 13 Jan 2011, coll. F. P. L. Marques, W. P. Duncan (RN11.48); MZUSP 108455, Rio Negro, Amazonas, Barcelos, Ilha do Jacami, 0°58’48” S, 062°55’12” W, 24 Feb 2004, coll. F. P. L. Marques, M. L. G. Araújo (RN04.08); MZUSP 108456, Rio Negro, Amazonas, Barcelos, Ilha do Jacami, 0°58’48” S, 062°55’12” W, 29 Feb 2004, coll. F. P. L. Marques (RN04.54); MZUSP 108709 (cleared and stained), Rio Negro, Barcelos, Amazonas, 0°58’48” S, 062°55’12” W, Ilha Cuchara, 0 8 March 2004, coll. F. Marques (NR04.98); MZUSP 108708 (dissected for muscles, central nervous system, and ventral lateral line), Rio Itu, Barcelos, Amazonas, 0°58’48” S, 062°55’12” W, Boca do Igarapé Aduiá, 0 8 March 2004, coll. A. da Silva Campos (NR04.93). [Seven specimens collected but not preserved, all from Rio Negro, Amazonas, Barcelos, 0°58’48” S, 062°55’12” W, Jan 2005, coll. M. Domingues, N. Luchetti, M. L. G. Araújo (field nos. RN05.12, RN05.21, RN05.22, RN05.23, RN05.25, RN05.36, RN05.43)].
Diagnosis. A species of Potamotrygon distinguished from all congeners by its unique dorsal color pattern, composed of a dark brown, black or olive background color forming a reticulate pattern, with numerous yellow, light brown or creamy colored cerebral or rosette-like designs over disc and base of tail region; these designs may contact each other but do not form a highly intricate vermiculate pattern. Potamotrygon schroederi is distinguished from all species of Potamotrygon , except P. t i g r i n a, by presenting the distal half of tail, posterior to caudal sting origin, with a sharply contrasted banding of five to six solid dark brown to blackish bars and uniformly cream colored interspaces of about equal width. Additionally, P. schroederi can be distinguished from congeners, except P. tigrina , P. orbignyi , P. hu m e ro s a, P. histrix and P. m a r in ae, by presenting a single developed and relatively short angular cartilage (more or less one-third length of hyomandibula; congeneric species with two more or less equally developed angulars, or with a single highly developed angular that is close to one-half hyomandibula length). The specific pattern of dorsal tail spines (usually low and well separated, occurring in one to three undefined rows, and originating relatively posteriorly on tail) further separates P. schroederi from other Potamotrygon species, with the exception of P. tigrina (other congeners usually with a single or multiple row of dorsal tail spines, usually in well defined rows, with relatively taller tail and more closely packed spines, originating more anteriorly on dorsal tail region at tail base).
Description. Measurements are presented in Table 3 as both raw data in mm and transformed into % DW; meristic features are given in Table 4.
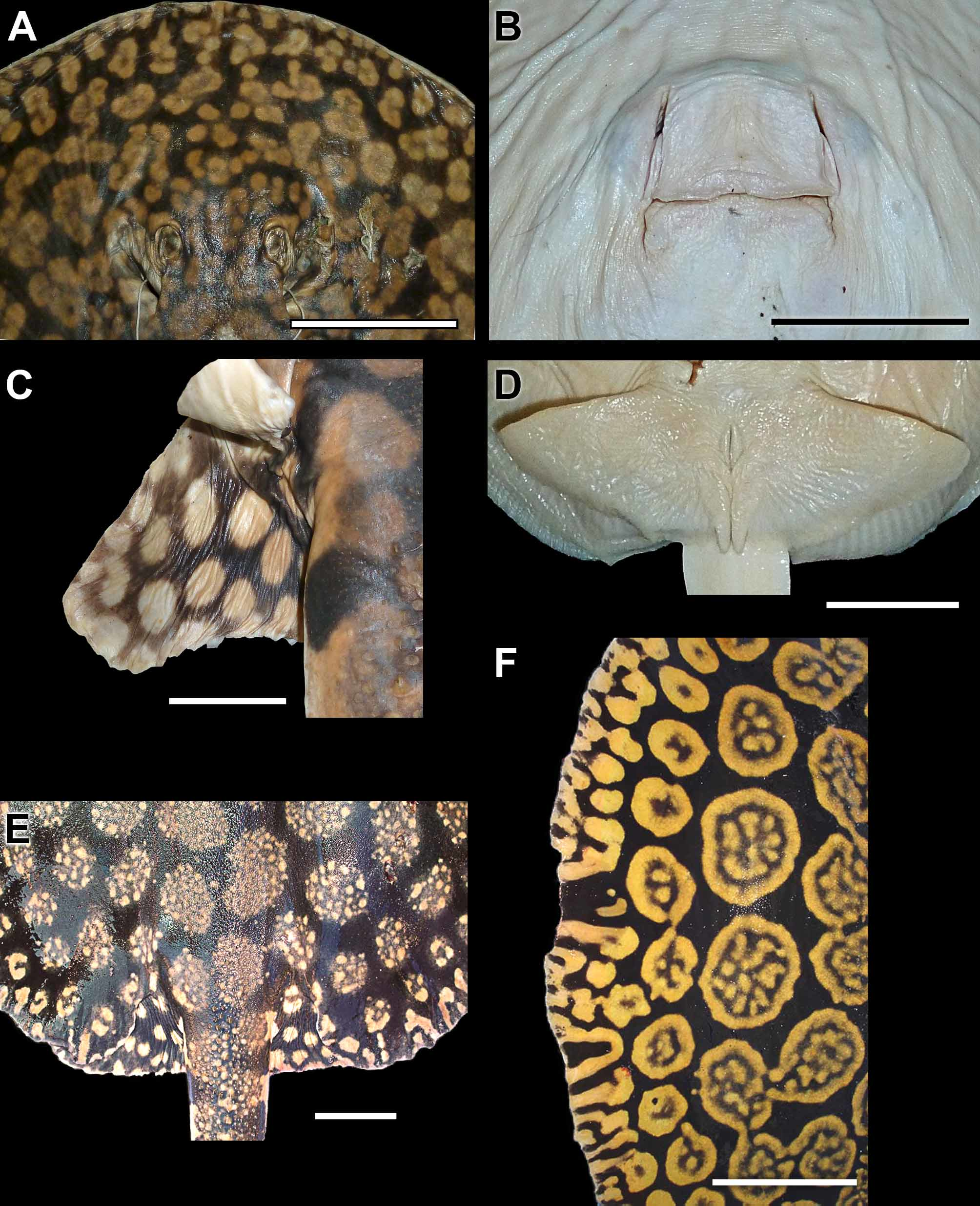
External morphology. Disc clearly oval, longer than wide; disc length ranging from 106 to 116.4% DW (x = 110% DW), and widest close to its midlength. Snout oval, with small rostral knob protruding from anterior disc in smaller specimens, but very reduced and barely noticeable in larger individuals. Preorbital snout length more than twice interorbital distance, ranging from 18.7 to 29.8% DW (x = 26.4% DW). Prenasal (14.1 to 19.9% DW, x = 17% DW) and preoral (19.0 to 25.2% DW, x = 22.5% DW) snout lengths relatively short, shorter than preorbital snout length. Eyes somewhat small, protruding from top of head region; eyes smaller than spiracles in diameter. Spiracles trapezoidal in fresh material, greatly expanded, but more contracted and slit-like in preserved specimens. Spiracles closely adjacent to eyes, about as wide as long in fresh material, and without elevated spiracular rims or central knob posteriorly. Spiracles extend anteriorly to level of one-half to posterior two-thirds of eye length in preserved material, but spiracles extend anteriorly to beyond eye length in live specimens. Interorbital distance slightly smaller than, or about equal to, interspiracular distance.
Mouth somewhat small, its opening relatively straight across in larger specimens, but somewhat arched in smaller individuals. Mouth width slightly less than one-half of distance between first gill slits, and slightly greater than internarial distance. Faint grooves present on posterior margins of lower jaw and outer corners of jaws due to preservation; labial folds absent. Nostrils elongated anteroposteriorly, slit-like, similar in length to eye diameter, and about one-half of internarial distance. Nasal curtain widest posteriorly, with posterior margin fringed and with only slight medial notch. Teeth not visible externally with mouth closed. Tooth rows in adult females ranging from 45–49/53–60, from 38–41/45–52 rows in preadult females, and 38–41/ 45–46 in juveniles ( Table 4; teeth also counted in specimens not in table). Teeth set in quincunx, relatively small, rhomboidal, and without conspicuous cusps. Five buccal papillae present inside mouth, lateralmost papillae much smaller in height. Branchial basket longer than wide, its length ranging from 16.5 to 18.6% DW (x = 17.6% DW). Ventral gill region relatively wide, distance between first and fifth gill slits about equal to distance between first pair of gill openings; distance between first gill slits slightly greater than distance between fifth gill slits. Gill flaps slightly undulated; fifth gill slit smallest, and slightly more obliquely positioned.
Table 3. Measurements of specimens of Potamotrygon schroederi from Venezuela (Orinoco basin) in A–C and Brazil (Negro basin) in D–F. A: Holotype from Fernandez-Yépez, 1958). B: AUM 44507 (adult male). C: ANSP 191999 (adult female). D: MZUSP 108453 (adult female). E: MZUSP 108452 (adult female). F: MZUSP 108456 (juvenile male). N: number of specimens measured; x: mean; SD: standard deviation. Note that holotype (A) is not included in range, mean or SD. * measurement not given in original account.
PARAMETER A B C D E F range x SD
Mm % DW mm % DW mm % DW mm % DW mm % DW mm % DW % DW N mm % DW mm % DW total length (TL) 995.0 – 780.0 – 605.0 – 689.0 – 741.0 – 320.0 – – 16 492.1 – 209.6 – disc length (DL) 600.0 114.5 470.0 111.9 395.0 110.3 444.0 108.6 444.0 112.4 181.0 106.5 106.0 – 116.4 16 301.8 110.4 128.2 3.0 disc width (DW) 524.0 100.0 420.0 100.0 358.0 100.0 409.0 100.0 395.0 100.0 170.0 100.0 – 16 273.1 100.0 113.3 0.0
16
interorbital distance 67.0 12.8 47.5 11.3 42.5 11.9 48.0 11.7 48.4 12.3 18.0 10.6 8.2 – 15.3 32.9 12.0 14.4 2.0 interspiracular distance 65.0 12.4 61.5 14.6 51.7 14.4 49.0 12.0 50.6 12.8 30.0 17.6 12.0 – 17.6 16 37.1 13.9 13.9 1.6 eye length * – 13.4 3.2 12.5 3.5 13.0 3.2 10.6 2.7 12.0 7.1 2.7 – 7.1 16 9.9 4.1 2.4 1.1 spiracle length * – 37.0 8.8 28.0 7.8 33.8 8.3 36.0 9.1 11.0 6.5 6.0 – 9.1 16 20.3 7.7 9.0 0.9 preorbital length 160.0 30.5 113.2 27.0 101.0 28.2 106.3 26.0 105.0 26.6 41.0 24.1 18.7 – 29.8 16 73.9 26.8 34.1 2.7 prenasal length * – 60.8 14.5 56.3 15.7 61.8 15.1 61.6 15.6 24.0 14.1 14.1 – 19.9 16 42.2 16.8 13.0 1.7 preoral length * – 80.9 19.3 73.6 20.6 77.9 19.0 83.6 21.2 35.0 20.6 19.0 – 25.2 16 55.8 22.2 16.9 1.9 internarial distance * – 33.5 8.0 24.9 7.0 27.9 6.8 26.8 6.8 12.0 7.1 6.5 – 8.0 16 18.2 7.0 7.3 0.5 mouth width * – 32.0 7.6 24.6 6.9 36.0 8.8 38.2 9.7 11.0 6.5 5.0 – 9.7 16 19.1 7.2 9.5 1.2 distance between 1st gill slits * – 105.2 25.0 86.1 24.1 84.9 20.8 89.8 22.7 39.0 22.9 18.7 – 25.0 16 56.1 21.6 23.7 1.7 distance between 5th gill slits * – 67.8 16.1 57.5 16.1 63.3 15.5 60.5 15.3 27.0 15.9 13.3 – 16.5 16 38.9 15.0 15.6 1.0 branchial basket length * – 82.3 19.6 64.9 18.1 71.6 17.5 73.5 18.6 31.0 18.2 16.5 – 19.6 16 46.1 17.8 18.4 0.7 pelvic fin anterior margin length 135.0 25.8 92.6 22.0 85.0 23.7 74.1 18.1 90.9 23.0 35.0 20.6 18.1 – 24.7 16 61.1 22.2 27.9 2.2 pelvic fin width 300.0 57.3 238.0 56.7 215.0 60.1 166.0 40.6 208.0 52.7 88.0 51.8 40.6 – 60.1 16 143.2 52.2 64.8 5.3 clasper external length * – 50.9 12.1 – – – – – – 6.0 3.5 1.2 – 12.1 3 19.6 5.6 27.2 5.7 clasper internal length * – 98.7 23.5 – – – – – – 20.0 11.8 0.6 – 23.5 3 39.9 12.0 51.8 11.4 distance between cloaca and tail tip * – 386.0 91.9 273.0 76.3 300.0 73.3 379.0 95.9 166.0 97.6 59.6 – 98.3 16 218.9 85.7 83.6 10.5 tail width 68.0 13.0 66.5 15.8 50.6 14.1 53.7 13.1 49.9 12.6 31.0 18.2 9.0 – 18.2 16 35.0 12.5 17.6 2.3 snout to cloaca distance 502.0 95.8 403.0 96.0 316.0 88.3 354.0 86.6 368.0 93.2 142.0 83.5 83.5 – 96.0 16 246.4 89.5 109.4 3.5 pectoral to posterior pelvic length * – 55.8 13.3 37.6 10.5 56.0 13.7 55.0 13.9 16.0 9.4 8.2 – 18.8 16 31.8 12.1 14.6 2.7 distance from cloaca to sting origin * – 236.0 56.2 171.0 47.8 233.0 57.0 246.0 62.3 77.0 45.3 39.4 – 62.3 14 133.3 50.0 66.6 6.0 sting length 97.0 18.5 72.1 17.2 74.8 20.9 48.0 11.7 82.8 21.0 32.0 18.8 11.7 – 21.8 14 48.0 17.5 23.1 3.3 sting width * – 7.3 1.7 5.5 1.5 4.9 1.2 5.0 1.3 3.0 1.8 1.0 – 1.9 14 3.8 1.5 1.5 0.3 mens from Brazil (Negro basin) in C–J. A: AUM 44507 (adult male, 780 mm TL). B) ANSP 191999 (adult female, 605 mm TL). C:
MZUSP 108452 (adult female, 444 mm DL). D: MZUSP 108453 (adult female, 444 mm DL). E: MZUSP 108445 (preadult female,
320 mm DL). F: MZUSP 108447 (preadult female, 305 mm DL). G: MZUSP 108448 (preadult female, 249 mm DL). H: MZUSP
108455 (juvenile male, 202 mm DL). I: MZUSP 108451 (juvenile female, 193 mm DL). J: MZUSP 108456 (juvenile male, 181 mm
DL).
CHARACTER A B C D E F G H I J range mode precaudal vertebrae 28 27 31 27 29 30 29 25 29 31 25–31 29 caudal vertebrae 124 122 99 100 96 100 88 81 104 98 81–124 100 total vertebrae 152 149 130 127 125 130 117 106 133 129 106–152 130 diplospondylous vertebrae 120 119 96 98 95 96 84 77 101 92 77–120 96 upper tooth rows 43 44 50 55 41 38 47 39 40 38 38–55 38 lower tooth rows 50 51 60 59 52 45 52 48 49 45 45–60 52 propterygial radials 40 40 42 41 38 40 39 40 41 45 38–45 40 mesopterygial radials 17 18 17 16 17 17 16 16 17 16 16–18 17 metapterygial radials 38 35 35 35 34 36 34 37 34 34 34–38 34 total pectoral radials 95 93 94 92 89 93 89 93 92 95 89–95 93 pelvic radials 24 26 24 22 20 19 24 21 19–26 24
Pelvic fins somewhat short, much wider than long (ranging from 40.6 to 58.0% DW, x = 50.9% DW), and broadest at more or less their anterior third. Apices of pelvic fins broadly oval; posterior margins of pelvics undulated. Pelvic fins mostly concealed in dorsal view. Tail relatively wide at base, its width about equal to interorbital distance (tail width ranging from 9.0 to 18.2% DW, x = 12.1% DW). Tail tapering posteriorly, but not resembling an elongated whip distally. Ventral tail flat in cross-section. Lateral tail folds slender, ridge-like in adults, wider in juveniles, and originating at about base of tail, extending to more or less level of caudal stings; ridge-like dorsal and ventral tail folds present, originating posterior to caudal stings and extending caudally for a short distance. Dorsal and ventral tail folds anteriorly wider, extending posteriorly as a very shallow ridge. Length from cloaca to distal tail variable, ranging between 59.6 and 98.3% DW (x = 85.9% DW), much smaller than disc length, but similar to snout to cloaca length (varying from 83.5 to 94.0% DW, x = 88.7% DW). Caudal sting varying greatly in length, ranging from 11.7 to 21.8% DW (x = 17.2% DW), and much greater than interorbital distance or tail width at base. Caudal sting with posteriorly directed, sharp serrations on each side, terminating in a very acute point, and positioned posterior to tail mid-length.
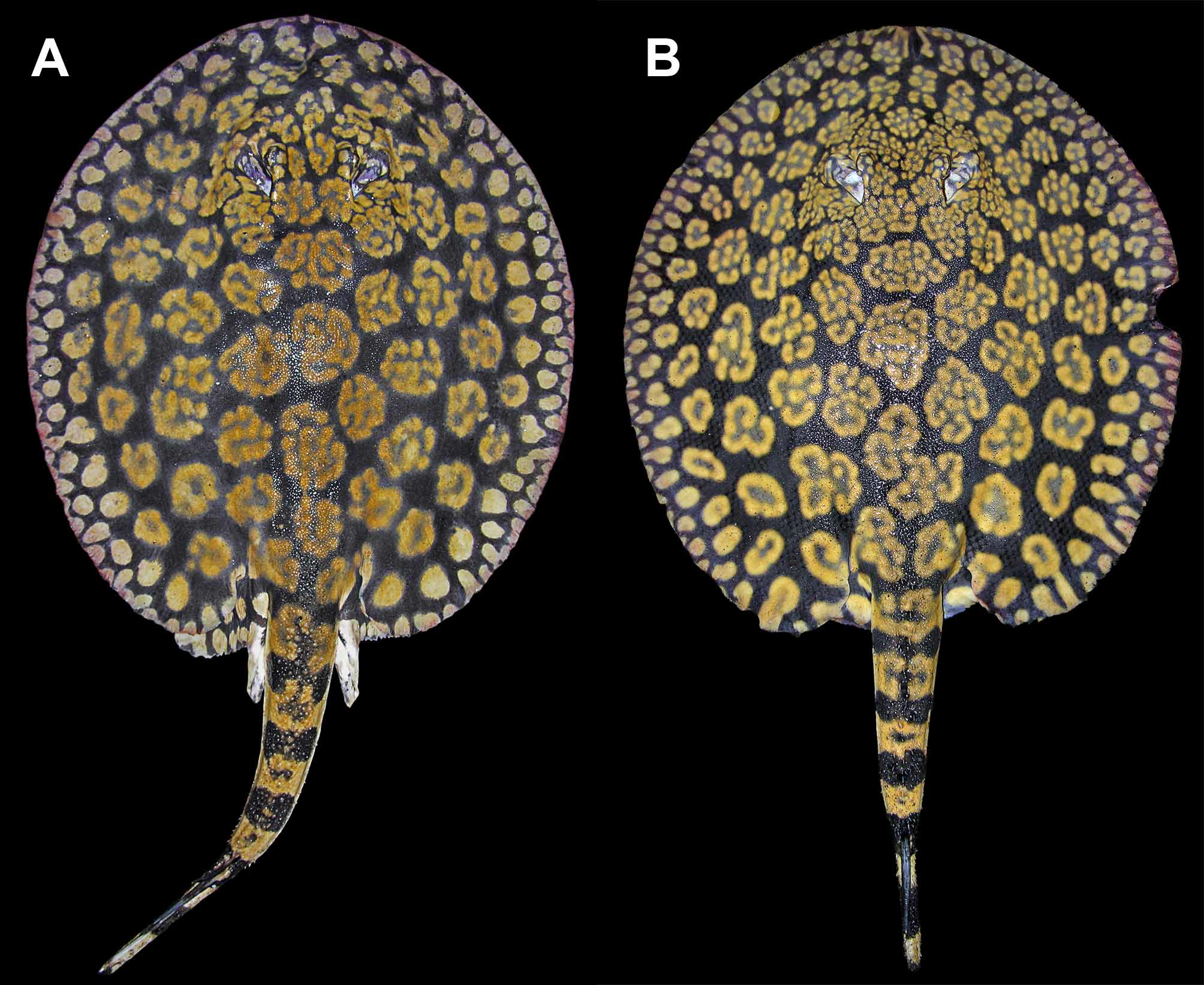
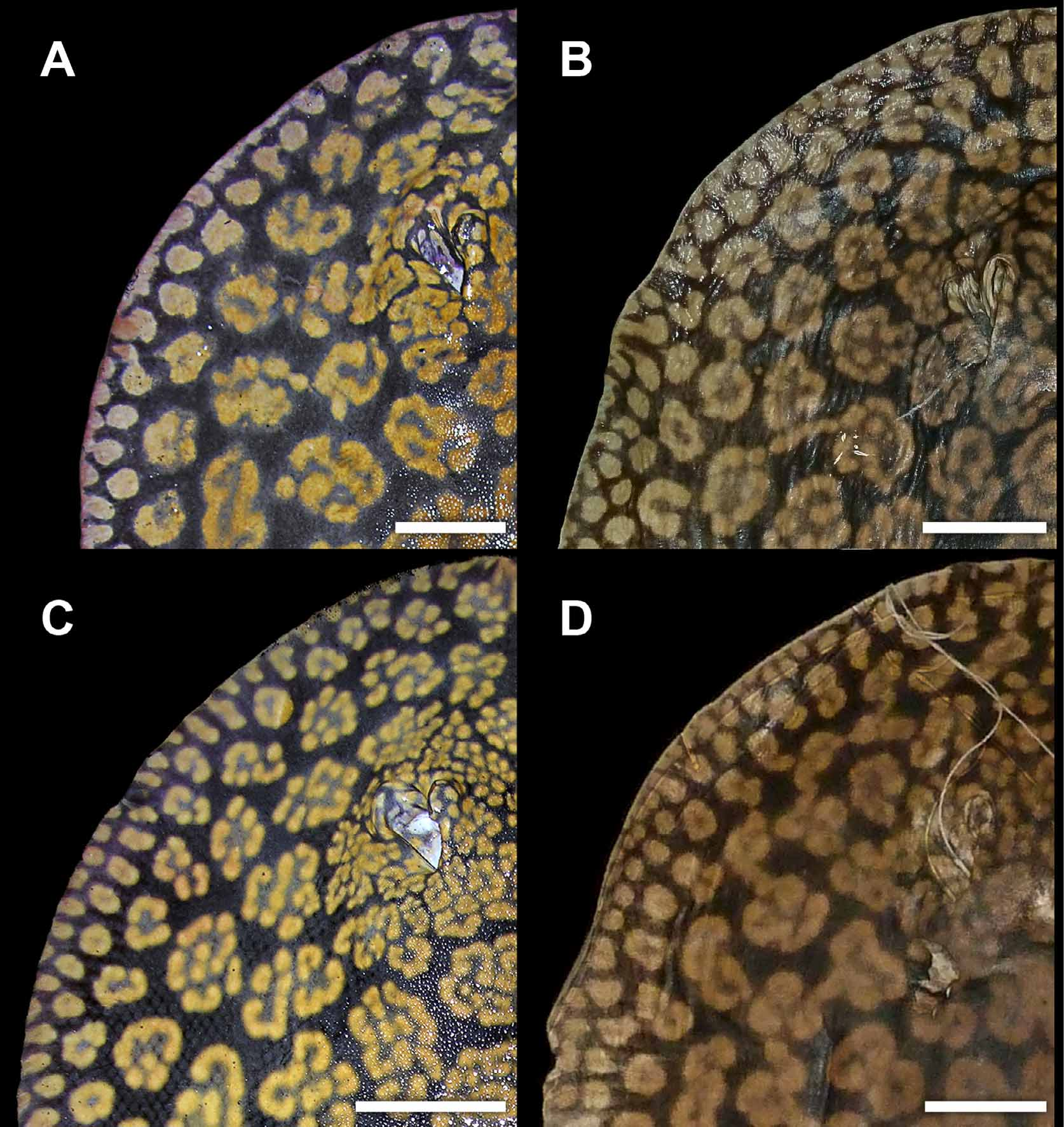
Coloration. Dorsal surface of disc with a gray, olive, dark brown to purplish-black background color, covered by numerous conspicuous tan, beige, golden yellow or orangish rosettes and brain-shaped figures over disc and anterior dorsal tail region ( Figures 10–16 View FIGURE 10 View FIGURE 11 View FIGURE 12 View FIGURE 13 View FIGURE 14 View FIGURE 15 View FIGURE 16 , 19 View FIGURE 19 ). Background color becomes slightly lighter toward outer disc margin. Outer disc with numerous spots much smaller than rosettes, more or less equal to eye diameter. These spots may be elongated, oval or appear as streaks of lighter color. Dorsal aspect of pelvic fins with beige to golden spots, smaller toward disc margins and larger at central pelvic fins region. Elaborate rosette-like figures on dorsal disc and anterior tail region formed by numerous smaller, lighter spots grouped into irregular circular, oval, clover or brain-shaped figures with lighter color in center. Size and particular pattern of elaborate rosettes vary from larger and more convoluted in disc center to smaller and less invaginated toward outer disc; markings very tightly associated, with more elaborate and finest convoluted pattern on interorbital region and posterior to spiracles on middisc. Dorsal rosettes and brain-shaped figures do not merge to form more conspicuous vermicular patterns, except over head and interorbital regions where finer, more slender rosette outlines usually occur. In specimens from the Orinoco basin, rosette outlines more elaborate. Smaller individuals with rosettes and brain-shaped figures proportionally smaller and more simple, with fewer convolutions, resembling ocelli with darker centers. Rosettes and brainshaped figures may form ocelli-like markings with darker central color in adults, but more restricted to outer and posterolateral disc surface. Anterior to mid-dorsal tail region with saddles of lighter color formed from somewhat incomplete or simple rosettes. Dorsal and lateral tail with strong banded pattern of alternating darker and lighter bars from caudal sting origin to posterior tail extremity. Ventral coloration uniform white to creamy-white, without conspicuous markings, but darker posterior margins of disc and pelvic fins sometimes present, especially in smaller individuals. Ventral tail with laterally appearing darker bands continuous from dorsal and lateral tail; these bands do not connect at ventral tail midline, more noticeable in smaller specimens. Posterior tail region as of caudal stings with alternating dark and lighter bands present; tail extremity dark. Live specimens with more intense color.
Dermal denticles. The dermal covering in P. schroederi is very similar to P. t ig r in a (see above). Dermal denticles present over central disc and dorsal tail base regions, composed of small denticles more or less evenly distributed. Denticles slightly greater and more spaced apart over middisc area, continuing posteriorly to base of tail. Base of dorsal tail with slightly enlarged, flattened, asterisk-shaped denticles. Denticles over midline of disc and tail with three or more crown dichotomies converging on crown apex. Dorsal tail spines in one to four very irregular rows. Dorsal tail spines smaller in juveniles, but increasingly taller, very straight in lateral view, and with robust bases in adults. Dorsal tail spines widely spaced apart ( Figures 16 View FIGURE 16 , 18 View FIGURE 18 ). Smaller spines occur in between taller dorsal tail spines. Dorsal tail spines originate relatively posteriorly on dorsal tail, posterior to, or more or less at level of, disc apices. Lateral tail region, posterior to or level with caudal stings and extending to distal tail extremity, with small, sharp denticles, especially in large adults.
Skeletal features. Skeleton very similar to P. tigrina . Neurocranium elongate, widest at nasal capsules and postorbital processes ( Figure 17 View FIGURE 17 ). Nasal capsules much wider than long, broadly rounded; internasal septum slen- der. Preorbital processes slightly wider than postorbital processes. Precerebral and frontoparietal fontenellae about fourth-fifths of neurocranial length; very short otic roof posteriorly at parietal fossa. Supraorbital process sharply triangular. Preorbital processes slender and acute distally. Antorbital cartilage very elongate and triangular, extending posteriorly to close to angular cartilage. Stout Meckel’s cartilage with dorsally projecting lateral process well developed; palatoquadrate much more slender. Hyomandibulae relatively slender and only slightly curved anteriorly toward midline. Anterior angular cartilage well developed, about one-third to one-fourth length of hyomandibula, with concave anterior margin; posterior angular apparently absent. Elongate cervicothoracic synarcual, about as wide as neurocranium, with about 30 spinal nerve foramina. Thoracolumbar synarcual slender. Individual vertebral centra occurring posterior to level of caudal sting origin ( Figure 18 View FIGURE 18 ); somewhat calcified notochordal extension (cartilaginous rod) present distally. Transition from mono- to diplospondyly occurs at about fourth centra posterior to pelvic girdle. Propterygium widest posteriorly, much more stout than meso- and metapterygium. Metapterygium with four posterior segments. Anterior segment of propterygium elongate, about equal in length to nasal aperture. Mesopterygium slightly convex externally. Pectoral radial elements sometimes fused at base, especially at posterior propterygium. Pectoral radials slender close to pectoral basals, slightly widening and shortening toward middisc, and slender again distally; pectoral radials subdivided at 9th or 10th segment. Pelvic girdle concave anteriorly lateral to low prepelvic process. Iliac processes extending caudally just beyond triangular ischial processes. Three to four obturator foramina present. Basipterygium stout, short, but longer than posterior metapterygial segments. Enlarged pelvic radial articulating with iliac process.
Remarks. There is some confusion regarding the year of publication of this species. The particular issue of the Boletin del Museo de Ciencia Naturales where P. schroederi was described refers to the years 1956 and 1957 on the inside and outside covers, but only in terms of accumulating papers accepted from those years. This same issue has printed on the inside cover “Junio de 1958” (June of 1958), and “Noviembre de 1958” (November of 1958) on the outside cover. In any case, this is indication that this particular issue was published in 1958 and not in 1957. Potamotrygon schroederi has been dated from 1957 by almost all previous authors (e.g. Castex & Yagolkowski, 1970; Rosa, 1985; Compagno, 1999, 2005; Carvalho et al., 2003; Rosa & Carvalho, 2007; Rosa et al., 2010).
The original description of P. schroederi is very brief and does not provide many morphological details ( Fernandez-Yépez, 1958). The description was based on a single very large female specimen (995 mm TL, 600 mm DL, 524 mm DW; Table 2), of which three small fragments remain (holotype, MHNLS 2504; Figure 9 View FIGURE 9 ). Fortunately, P. schroederi is very distinctive, and identifying these remains as P. schroederi is unequivocal, especially the distal caudal extremity and outer disc fragments (both have the typical coloration of P. schroederi ). The fragment representing the outer margin of the disc reveals the coloration in the holotype to be typical of larger, older individuals; a large examined specimen, smaller than the holotype, has a dorsal pattern with rosettes slightly more separated and with less defined margins, with rosettes containing numerous spots (cf. Figures 9 View FIGURE 9 C, 12A, 15E).
Fernandez-Yépez had briefly characterized P. schroederi , without naming it, in a previous publication (as “raya guacamaya”; Fernandez-Yépez, 1949) based on a different specimen from the holotype (from Puerto Carreño, Río Orinoco), which constitutes the first record and photograph of this species. In the original description, P. schroederi is considered as being most similar to P. m o to ro, contrasting it to Disceus thayeri Garman, 1913 [= Paratrygon aiereba (Müller & Henle, 1841) ] and “ P. hystrix ” [= P. histrix (Müller & Henle, 1834) , a species previously thought to be more widespread in the Amazon basin, but which is restricted to the Paraná-Paraguay system; Carvalho et al., 2003; Carvalho, unpubl.]. Fernandez-Yépez (1958) characterized P. schroederi as lacking oral papillae, but these are present in our material.
Castex & Yagolkowski (1970) extended the distribution of P. schroederi to Brazil, citing a large (720 mm TL) female specimen from Manaus. This specimen, however, most probably arrived in Manaus from the mid to upper Rio Negro. Castex & Yagolkowski (1970) also cite another specimen from the Orinoco from close to the typelocality, and publish the same figure present in Mago-Leccia’s (1970) account. They stated that P. schroederi , based on coloration, “low disc” and tooth pattern (among other ambiguous features), can be placed in a group with P. brachyura (Günther, 1880) , P. falkneri and P. menchacai . Even though their redescription did not provide many informative details, at least specimens from the Orinoco and Negro basins were compared for the first time.
The only more or less consistent distinction, based on our material, between populations of P. schroederi from the Rio Negro and Río Orinoco is in numbers of vertebral centra. Total vertebral centra in the two specimens from the Orinoco that were radiographed are clearly higher (149–152) than in the Rio Negro material (106–133), reflecting a much higher number of caudal centra (122–124 vs. 81–104, respectively). But our radiographs of material from the Orinoco were much more clear and easy to interpret, which could be partially responsible for such a significant difference. Also more material from Venezuela should be examined to corroborate this character.
Another difference, but more slight, occurs in dorsal coloration. Freshly collected specimens from both Rio Negro and Río Orinoco have a strong blackish-brown or purplish-black background color, and the rosettes and other dorsal ornamentation are also similar in intensity and brightness. But material from Rio Negro have relatively smaller and more numerous rosettes and brain-shaped vermiculate figures on dorsal disc region, especially on outermost rows closer to disc margin and on middisc. The specific patterns formed by these vermiculate figures also appear slightly distinct. In material from the Orinoco, the rosettes, as well as being slightly larger and fewer (especially on middisc region, close to midline), are more complex and invaginated ( Figures 10 View FIGURE 10 , 12 View FIGURE 12 , 19 View FIGURE 19 ) (note that these distinctions are difficult to quantify as counting individual rosettes is not straightforward). In material from the Orinoco, many rosettes and brain-shaped figures are clearly greater in diameter than interorbital space, whereas in specimens from Rio Negro, usually just a few of these figures, at the most, will surpass interorbital space in width. We have verified these patterns on specimens of similar dimensions and sex. Even though we have included here only two collected specimens from the Orinoco, we have seen others (also Castex & Yagolkowski, 1970; Mago- Leccia, 1970; numerous specimens in aquarium literature). Clearly, though, more material is needed to verify these distinctions.
Even though P. schroederi is known from two major river basins (Negro and Orinoco) spanning a large geographical area, this species can be easily identified from congeners; however, the distinctions among populations of both basins outlined above are either relatively minor or require further corroboration, and at present do not warrant specific separation. Both rivers are interconnected by the Río Casiquiare in Venezuela (for a recent summary, see Winemiller et al., 2008), but tagging studies are needed to verify if specimens traverse from one basin to the other, or if P. schroederi occurs without significant discontinuities between both basins; in any case this biogeographical link explains the historical distribution of P. schroederi in the Negro and Orinoco basins.
According to Araújo (2004), specimens of P. schroederi are born at 140 mm DW, mature sexually at 420 mm DW (males) and 440 mm DW (females), and attain a maximum size of 540 mm DW (dimensions similar to P. tigrina ). This species is commonly exported by the aquarium trade from Manaus.
Geographic distribution. Potamotrygon schroederi is distributed in the mid to upper Rio Negro and mid to upper Río Orinoco basins ( Figure 8 View FIGURE 8 ). This species appears to prefer faster flowing waters of central river channels, and has not been captured in the lower Rio Negro (M. L. G. de Araújo, pers. comm.). Specimens from the upper Río Ventuari (ANSP 191999) and Río Orinoco (AUM 44507) were collected at night in a swift current (under 2 m deep), over a large beach of coarse sand. This species probably also penetrates the region of the Río Casiquiare interchange between the Negro and Orinoco basins. Reported also from the Orinoco basin of Colombia, but specimens were not examined by us and are not illustrated in accounts ( Mejía-Falla et al., 2007; Maldonado-Ocampo et al., 2008).
Common name. Flower ray (from aquarium literature).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Potamotrygon schroederi Fernandez-Yépez, 1958
| De, Marcelo R., Sabaj, Mark H. & Lovejoy, Nathan R. 2011 |
Potamotrygon schroederi Fernandez-Yépez (1958
| Fernandez-Yepez 1958 |