Rhynchelmis (Sutroa) klamathensis Fend
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3760.2.3 |
|
publication LSID |
lsid:zoobank.org:pub:587163F9-326C-4CBC-94A5-4605C8ADB000 |
|
DOI |
https://doi.org/10.5281/zenodo.6139328 |
|
persistent identifier |
https://treatment.plazi.org/id/0397878B-FF8D-FFAB-FF4D-5DB1FB52FEC0 |
|
treatment provided by |
Plazi |
|
scientific name |
Rhynchelmis (Sutroa) klamathensis Fend |
| status |
sp. nov. |
Rhynchelmis (Sutroa) klamathensis Fend View in CoL n. sp.
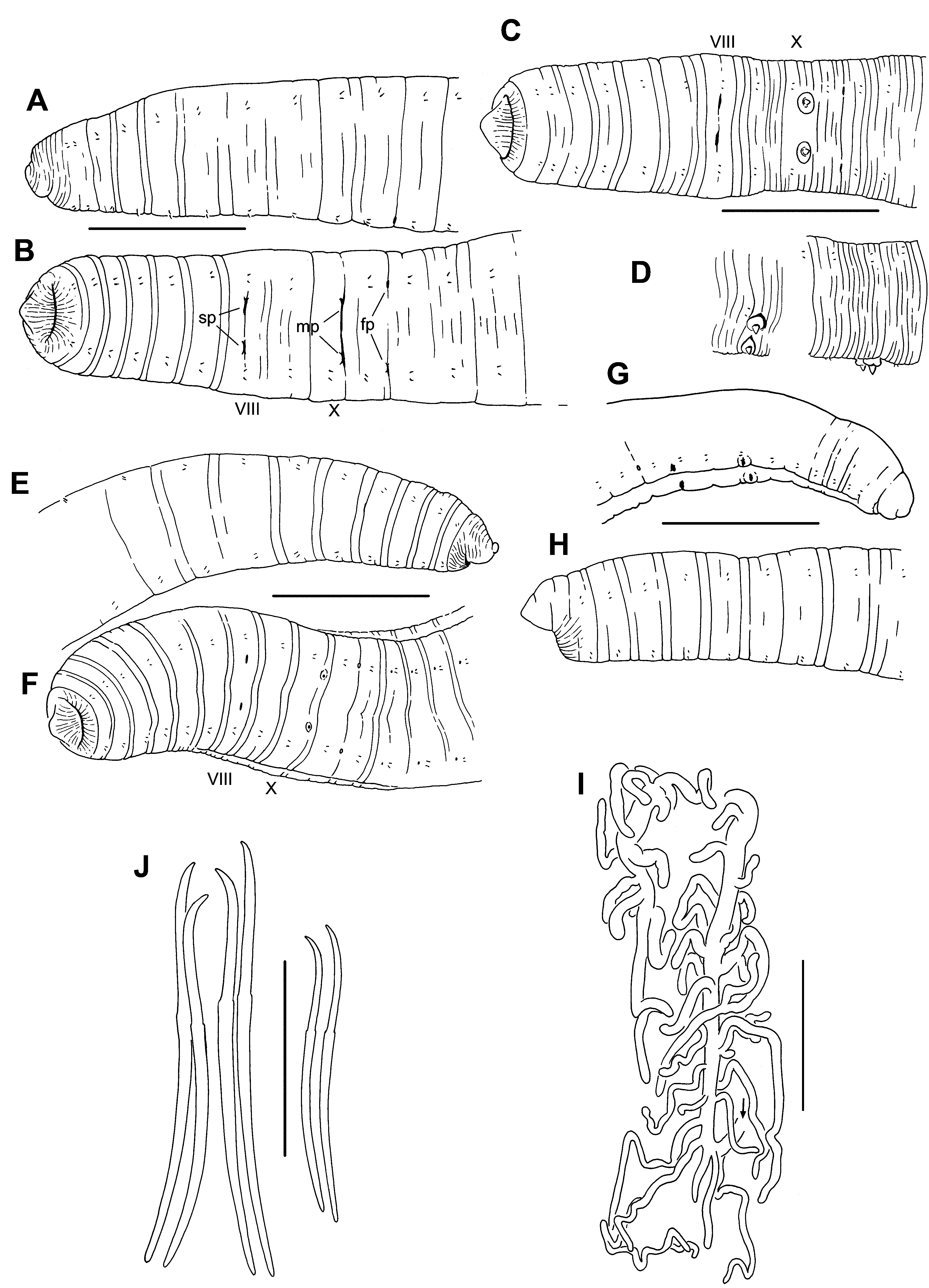
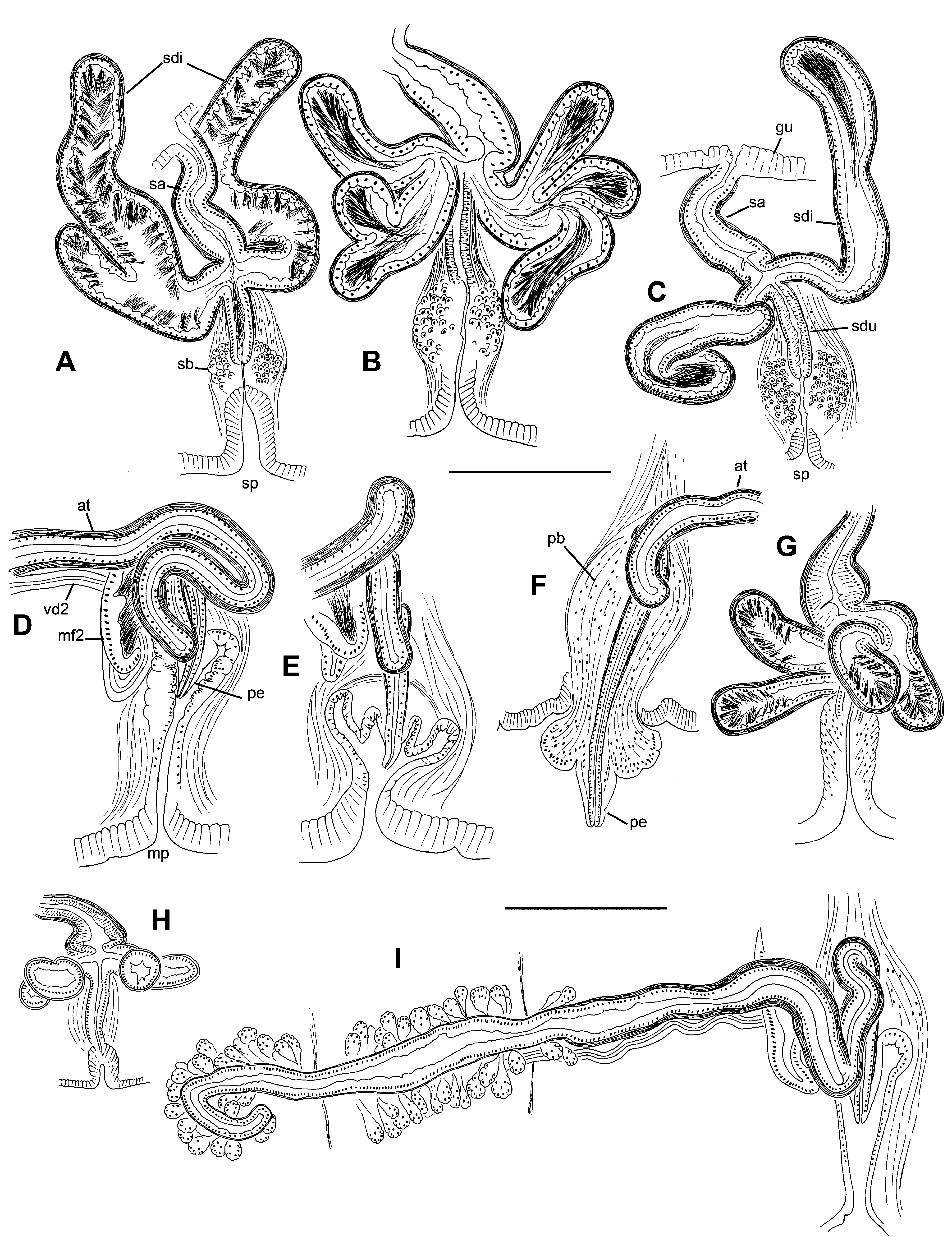
( Figs 2–5 View FIGURE 2. R View FIGURE 3. R View FIGURE 4. R View FIGURE 5. R )
Holotype. USNM USNM 1230305, a dissected, slide-mounted specimen.
Type locality. OREGON, Klamath Co.: Upper Klamath Lake at Modoc Rim (eastern side of lake, south of confluence with Williamson River), N42.415 °, W121.873 °, 24 Sep 2008.
Paratypes. USNM 1230306-1230310, the type locality, 8 Oct 2008, 3 dissected on slides; 10 Sep 2008, 2 sectioned worms (1 transverse, 1 sagittal), 1 dissected on slide. Upper Klamath Lake at Williamson River mouth (northern end of lake), 6 Nov 2008, 2 dissected. CASIZ 192763-192765: the type locality, 10 Sep 2008, 1 dissected; 24 Sep 2008, 1 dissected; 6 Nov 2008, 1 dissected.
Other material. OREGON, Klamath Co.: slide-mounted, mature specimens. Upper Klamath Lake at Modoc Rim, 10 Sep 2008, 4 dissected, 1 whole mount, 1 sagittally sectioned; 8 Oct 2008, 4 dissected; 6 Nov 2008, 3 dissected, 1 sagittally sectioned. Upper Klamath Lake at Williamson River, 6 Nov 2008, 2 dissected. Upper Klamath Lake near entrance to Ball Bay, 3 Oct 2011, 4 dissected. Unmounted, mature specimens: various open-water sites in northern part of lake:, 27 Aug 2008 (4); 10 Sep 2008 (17); 17 Sep 2008 (8); 24 Sep 2008 (1); 8 Oct 2008 (11); 6 Nov 2008 (15); 1 Sep 2009 (24); 31 Aug 2010 (17); 3 Oct 2011 (2). Immature and partially mature specimens, all unmounted: 2008: approximately weekly intervals from 1 May-8 Oct and 6 Nov. 2009: 9 Jun, 21 Jul, 1 Sep. 2010: 4 May, 8 Jun, 20 Jul, 31 Aug. 2011: 31 May, 27 Jul. Upper Klamath Lake material was collected by M. Lindenberg, A. Dolan-Caret, J.L. Carter, S.V. Fend, and J. Kuwabara. CALIFORNIA, Sacramento Co.: South Fork of the Mokelumne River, just west of the Tower Park Bridge on Highway 12, N38.1162 °, W121.5040 °, collector D. Riordan. 21 Oct 2009, 4 mature, dissected, on slides; 22 Jan 2010, 2 immature, whole mounted; 7 Oct 2010, 1 mature and 2 partially-mature, all dissected.
Etymology. The specific epithet refers to the type locality.
Description. Klamath Lake material, type locality. Length of 20 intact, preserved specimens 80–142 (103) mm; 245–340 (304) segments. Width 1.3–2.1 (1.7) mm in X (n=98), maximum width 1.4–2.6 (2.1) mm. Prostomium short, with a narrow, slightly upturned, conical tip; body roughly cylindrical ( Fig. 2 View FIGURE 2. R A–F). Segment I usually finely striated. External segmentation variably developed; each segment posterior to II usually with a weak secondary annulation at about the anterior 1/4, clitellum occasionally with many fine, transverse grooves ( Fig. 2 View FIGURE 2. R C). Clitellum usually from mid-IX to mid-XIV. Chaetae narrowly paired, with bundles approximately equidistant ( Fig. 3 View FIGURE 3. R D, E). Chaetae simple-pointed, sigmoid, with a moderately developed distal hook; nodulus distal to midpoint, 33– 41% of chaeta length from tip ( Fig. 2 View FIGURE 2. R J). Chaeta length 210–440 µm in middle segments, somewhat smaller in posterior segments; dorsals about as long as ventrals. Both male and spermathecal pores conspicuous. Longitudinal position of paired spermathecal openings at level of ventral chaetae in VIII; transverse position distinctly median to ventral chaetae ( Fig. 2 View FIGURE 2. R B, C). Male pores posterior and slightly median to ventral chaetae in X. Female pores at 11/ 12, in line with ventral chaetae.
Epidermis 14–29 µm thick in anterior segments, maximum thickness 35–89 µm in clitellum. Body wall longitudinal muscle layer 50–90 µm thick in preclitellar segments; circular muscle layer 13–22 µm. Longitudinal muscles divided at chaetal and lateral lines into bands which do not curl strongly inward at the edges. Brain in the peristomium; circumpharyngeal connectives join ventral nerve cord in II. Pharynx mostly in II–V, dorsal and ventral sides appear folded, without a distinct dorsal pad; walls about 30 Μm thick, with narrow, columnar cells. Pharyngeal glands usually in IV and V, sometimes extending into III or VI.
Ventral blood vessel divides at 6/7 or in VII. A pair of convoluted lateral blood vessels join both the dorsal and ventral blood vessels in posterior part of anterior segments; in II–VII, at least, they are unbranched, and chloragogen covers them only adjacent to the dorsal vessel; in VIII through about XIII they are covered with chloragogen cells in the dorsal half, and may have a few small branches near the dorsal vessel. In X and XI these vessels loop into the sperm and egg sacs, respectively, and in XII they loop into the septal sac (from 12/13) containing the sperm/egg sacs. Beginning about XV the posterior lateral blood vessels are extensively branched, covered by chloragogen, and join the dorsal vessel only; but posterior to about XXXV–XL a branch from each posterior lateral vessel joins the gut midlaterally. Beginning in VIII, a second pair of branched lateral blood vessels, covered with chloragogen cells, joins the dorsal vessel in the anterior part of each segment; posterior to XII, a thin branch from each anterior lateral vessel joins the perivisceral sinus on the ventrolateral aspect of the gut ( Fig. 2 View FIGURE 2. R I). Segments XI to about XX have 2–3 small, globular organs (“blood glands”, cf. Smith & Dickey 1918) at the perivisceral sinus, joined by small branches from the ventral blood vessel.
Nephridia usually from XIII, paired, single, or absent in posterior segments. A small anteseptal funnel is followed by an ovate, granular, postseptal mass about 80 Μm long, which narrows to a tubule; the tubule forms a closely paired loop, which closely follows the anterior lateral blood vessel dorsally to approach the dorsal blood vessel; the loop usually continues to follow other lateral vessel branches to the ventral vessel, and may continue to the posteroventral part of the segment. The ectal end of the tubule forms a short extension to a thin-walled, irregular vesicle about 80 Μm high, and an inconspicuous nephropore anterior to the ventral chaetal bundle.
Testes paired in X; vestigial testes usually in IX; ovaries paired in XI. Testes and ovaries relatively small, usually ending before mid-segment. Female funnels conical with posterior lip about 250 Μm high. Sperm sacs extend posteriorly as far as XXXI (median XXV) in mature worms, egg sacs extend a few segments further (median XXXI); no anterior sperm sacs. Paired septal cell masses, probably vestigial sacs, extend posteriorly from 8/9 and 9/10, into IX and X ( Fig. 3 View FIGURE 3. R A).
Spermathecae paired in VIII. Ectal duct of spermatheca tubular, composed of densely-packed epithelium, 125–230 (170) Μm long, diameter 32–53 (43) Μm; surrounded by a loose muscle layer about 25 Μm thick ( Fig. 5 View FIGURE 5. R B). Duct terminates within a “spermathecal bulb”, a bulb-shaped mass of irregularly arranged cells surrounding an invagination of the epidermis (which forms the external pore); bulb diameter 82–166 (132) Μm ( Fig. 5 View FIGURE 5. R A–B). Spermathecal ampulla tubular to narrowly pyriform, 205–400 (277) Μm long, maximum width 77–120 (91) Μm, with ental end opening into the ventrolateral side of the gut ( Figs 4 View FIGURE 4. R C, 5A). One pair of diverticula inserts at the junction of duct and ampulla, usually on the anterior and posterior sides ( Fig. 3 View FIGURE 3. R A). Spermathecal diverticula usually tubular ( Fig. 4 View FIGURE 4. R C), 500 (400–675) µm long by 106 (91–138) Μm in diameter; diverticula with 1 or 2 short branches in 5 specimens ( Fig. 4 View FIGURE 4. R A, B); diverticula unbranched in the holotype. A dense muscle layer surrounds ampulla and diverticula, thickness 7–10 Μm. Epithelium of diverticula and ampulla may be irregularly incised. Spermathecal sperm concentrated in diverticula, with heads lined up along epithelium ( Fig. 4 View FIGURE 4. R A–C); loosely arranged sperm also present in ampulla.
Male pores on tips of conical penes, usually retracted into convoluted sacs ( Figs 3 View FIGURE 3. R A, E; 4D, E). In most specimens, the inner end of the sac forms a low mound, which subtends the penis ( Fig. 4 View FIGURE 4. R E). In 1 specimen, the sacs are everted and the extruded mass of cells forms the base of the penis ( Figs 2 View FIGURE 2. R D, 4F). Penial sac surrounded by diffuse, bulb-shaped mass of tissue interspersed with muscle fibers ( Fig. 5 View FIGURE 5. R D), total height of bulb 360–500 Μm; entally the muscle fibers coalesce to form retractor muscles, joined to the dorsolateral body wall ( Figs 3 View FIGURE 3. R E, 5E). Atrial duct approaches penial bulb from median side and enters bulb from top-center. Atria tubular, 2310–3390 (2770) Μm long, usually highly contorted, extending 1–3 segments posteriorly. Atria about equally divided into an ectal, muscular portion, and an ental, prostate-bearing portion. Ectal portion 62–105 (80) Μm in diameter, with muscle coat 7–14 (11) Μm thick, and with regular, cuboidal epithelium ( Fig. 5 View FIGURE 5. R F, G). Ental part of atrium 50–82 (60) Μm in diameter, muscle layer 5–9 (7) Μm thick, covered with petiolate, multicellular prostate glands ( Fig. 5 View FIGURE 5. R H–I). Prostate glands about 70 Μm long, granular and containing small vacuoles; narrow ends penetrate atrial muscle. Anterior male funnels cup-shaped, 120–180 Μm high, without sperm; anterior vasa deferentia variably developed, sometimes appearing absent in X, not obviously ciliated, 12–26 (19) Μm thick. Posterior male funnels with sperm; highly convoluted, 220–400 Μm high, on 10/11, but sometimes extending back into sperm sac. Posterior vasa deferentia distinctly ciliated, 22–36 (30) Μm in diameter. Both vasa deferentia free within the sperm sac for most of their length ( Fig. 5 View FIGURE 5. R G–I); junction with the atria unclear in most specimens, but possibly variable; where visible, they join the atria in the middle to ental part of the prostate-covered portion.
Partially mature worms: Testes and ovaries may be large, extending throughout their respective segments. Spermathecal pore a shallow epidermal fold. Ectal duct and surrounding muscle layer of spermatheca are well developed before the ental duct joins the gut; prior to copulation, diverticula develop from a median expansion and the ental duct joins the gut. Penial sac develops gradually from an epidermal fold; muscular penial bulb develops before a penis is formed. Atrium tubular, coiled in X, with multicellular prostates forming on about the ental 3/5. Posterior male funnel large and convoluted before mature spermatozoa develop; in 1 specimen, posterior vas deferens approaches atrium near beginning of prostates ( Fig. 3 View FIGURE 3. R C), and anterior vas deferens joins atrium near ental end. Female funnels well developed.
Mokelumne River material. Length of a single intact, preserved specimen 48 mm, with 96 segments; a nearly complete specimen with 95 segments. Width 0.9–1.6 mm in X (n= 6), maximum width 1.1–1.7 mm. Prostomium shorter than wide, with a slightly narrowed tip ( Fig. 2 View FIGURE 2. R G, H). External characters as described above. Chaetae 240–336 µm in middle and posterior segments, somewhat smaller in posterior segments; dorsal pairs about as long as ventrals; nodulus 27–36% of chaeta length from tip. Pharyngeal glands in IV–VI. Blood vessels as described for worms from the type locality.
Sperm sacs extend posteriorly to as far as XXXI and egg sacs to XXXIII in 1 mature worm. Ectal duct of spermatheca 120–160 Μm long, diameter 46–52 Μm, surrounded by a loose muscle layer ( Fig. 3 View FIGURE 3. R B). Spermathecal bulb more weakly developed than in worms from the type locality, consisting of thickened epidermal cells; diameter about 100 Μm. Spermathecal ampulla distinctly widened at base to 110–145 Μm; ental duct width 45–50 Μm, with ental end opening into the lateral side of the gut; total length of ampulla and duct 180–230 Μm ( Fig. 4 View FIGURE 4. R G). Spermathecal diverticula each with 1–2 branches ( Figs 3 View FIGURE 3. R B, 4G–H), 260–330 µm long by 80–110 Μm in diameter. Muscle layer surrounding ampulla and diverticula about 6–10 Μm thick.
Male pores on narrowly conical penes ( Figs 3 View FIGURE 3. R B, 4I); penial bulbs and junction with atrial duct as described above. Atria about 1600–1900 Μm long, variably contorted, extending 1–3 segments posteriorly; ectal, muscular part slightly longer than ental, prostate-bearing portion ( Fig. 4 View FIGURE 4. R I). Ectal part of atrium 60–90 Μm in diameter, with muscle coat 8–12 Μm thick; ental part 55–80 Μm in diameter, muscle layer 5–8 Μm thick, covered with multicellular prostate glands 50–100 Μm long. Anterior male funnels 80–130 Μm high, without sperm; anterior vasa deferentia 18–19 Μm thick. Posterior male funnels with sperm; highly convoluted, 200–280 Μm high on 10/11, not extending back into sperm sac; posterior vasa deferentia 21–25 Μm in diameter. Vasa deferentia join the atria in the middle to ental part of the prostate-covered portion.
Remarks Most specimens of these fragile worms were incomplete; only 20 Upper Klamath Lake worms deemed mature or post-mature (based on diameter> 1.5 mm, at least vestigial genital pores or gonads, and dark chloragogen), and one Mokelumne River specimen, appeared to be intact. There were few specimens with obviously regenerating tails, suggesting that these worms do not commonly reproduce by autotomy, but readily fragment under stress.
The consistent lack of a filiform proboscis distinguishes R. klamathensis from all other Nearctic Rhynchelmis species. Differences with the sympatric R. rostrata are discussed above and in Appendix 3. Most R. klamathensis specimens resemble R. aleutensis and R. gilensis in having paired, unbranched spermathecal diverticula and elongate-tubular spermathecal ducts. Rhynchelmis aleutensis , known only from Adak Island, differs in several characters from R. klamathensis and other typical R. ( Sutroa ) species (Appendix 3). The tendency for R. klamathensis atria to form a convoluted mass, commonly terminating in the first postatrial segment, is unusual; most Rhynchelmis species have relatively straight atria which extend back through several segments. Rhynchelmis gilensis also has relatively short, convoluted atria, and conical penes. However, R. gilensis differs from R. klamathensis in having a single, median spermatheca, both vasa deferentia entering atria near the ental end, a filiform proboscis, and no lateral blood vessels in posterior segments. It is also a relatively small worm, known only from hyporheic habitats in Arizona and New Mexico.
The limited branching of spermathecal diverticula in some specimens suggests affinity with the “ yakimorum complex” (Appendix 3), a group associated with current or former Snake River drainages ( Zhou et al. 2010). The latter species consistently have multi-lobed spermathecal diverticula, but differ from R. klamathensis in having very short or indistinct spermathecal ducts. Rhynchelmis (Sutroa) specimens from springs in the downstream, Klamath River drainage have these characters, and were described as a variant of R. yakimorum ( Fend & Brinkhurst 2000) .
Somatic characters easily distinguish immature R. klamathensis from other large lumbriculids in the region. The lack of a filiform proboscis or modified anterior chaetae distinguishes the new species from Kincaidiana hexatheca Altman, 1936 . The short, slightly upturned prostomium will distinguish most specimens from typical Lumbriculus variegatus Müller , three western Eclipidrilus species, and Stylodrilus heringianus Claparède , all of which have a conical to rounded prostomium; the latter two taxa also tend to have a more tapered body form. The simple-pointed chaetae distinguish immature specimens from L. variegatus and S. heringianus . The two pairs of highly branched lateral blood vessels, beginning as far forward as VIII, are typical for Nearctic Rhynchelmis ; other lumbriculid genera may have two branched pairs in posterior segments, but western Nearctic species have at most one pair in the mid-body region.
The two populations attributed to R. klamathensis are geographically distant, and differ in size and in some morphological details. Mature worms from the Klamath population are unusually large for the genus, with many segments (commonly over 300), compared with about 100 segments in the most complete Mokelumne specimens. Other Nearctic Rhynchelmis species typically have between 100 and 150 segments ( Fend & Brinkhurst 2000). All of the Mokelumne specimens had branched spermathecal diverticula, whereas diverticula were unbranched in most Klamath specimens. The slightly shorter atria and somewhat less-defined spermathecal and penial bulbs of the Mokelumne population may also be real population differences, but might also be related to state of maturity or even differences in fixation.
Distribution and phenology. Upper Klamath Lake is a large (approximately 30 km by 12 km), shallow (mostly 2–3 m depth), freshwater lake in southern Oregon. The lake has been operated as a reservoir since 1917, eventually draining into the Klamath River, which flows to the Pacific Ocean. Elevation is 1262 m; climate is temperate with some ice cover from about December through April, and maximum summer temperatures near the bottom usually do not exceed 25°C ( Wood et al. 2006). The lake is naturally eutrophic, but is currently considered hypereutrophic, possibly as a consequence of marsh reclamation and agriculture in the surrounding watershed (e.g., Eilers et al. 2004). Annual blooms of the cyanobacterium Aphanizomenon flos-aquae (L.) Ralfs cause fluctuations in water chemistry, including localized periods of low dissolved oxygen during warmer months in bays and in a deep (approx. 15 m), narrow trench along the western margin. However, severe hypoxia appears to be uncommon in much of the shallow, open-water habitat ( Hazel 1969; Wood et al. 2006) and more recent summer-fall dissolved oxygen measurements were generally above 4 mg /L at sites where R. klamathensis was collected ( Kanarr et al. 2010). Upper Klamath Lake and its watershed are known to contain several endemic fishes ( NAS 2003) and mollusks ( Frest & Johannes 1995).
Within Upper Klamath Lake, R. klamathensis was only found in open water habitats, and appeared restricted to shallower parts of the lake (2–3 m depth), where soft, diatomaceous sediments predominate ( Hazel 1969). The new species was almost never collected in the trench, and nor was it found in streams in the surrounding watershed where the widespread R. rostrata occurred. It was uncommon at a littoral site, and occurred in low numbers in reclaimed parts of the Williamson River Delta only in the third year after flooding (2010).
Over 2000 specimens of R. klamathensis have been identified in the various studies; about 80 of these were mature. Densities of R. klamathensis at the eight stations where it was most abundant were about 80–800 (mean=460)/ m2. Although the species was numerically less abundant than several other benthic annelids, its large size made it a dominant component of the biomass based on a visual comparison of volume. The open-water lake macrobenthos was numerically dominated by the microdrile oligochaete families Naididae and Lumbriculidae , the freshwater sabellid polychaete Manayunkia Leidy , the hirudinean families Erpobdellidae and Glossiphoniidae , and the dipteran family Chironomidae . Less-abundant, but relatively large taxa included gastropods ( Planorbidae , Valvatidae and Hydrobiidae s. lat.) and pelecypods (primarily Pisidiidae ) (see also Kuwabara et al. 2012; Hazel 1969). As in the case of Rhynchelmis , some other common oligochaetes in the open-water lake habitat differ from more typical congeners in adjacent habitats within the watershed. The open-water form of Altmanella freidris (Cook) (Lumbriculidae) is distinctive in having much fewer body segments than typical specimens found at wetland and tributary sites ( Fend 2009). A distinctive, open-water Varichaetadrilus (Tubificinae) , possibly attributable to Varichaetadrilus pacificus (Brinkhurst) , lacks hair chaetae, and is much larger than the more typical specimens found near the lake margin, (J.L. Carter & S.V. Fend, unpublished). The two dominant glossiphoniid leeches in open-water habitats (both in the genus Helobdella ) differ from the widespread Helobdella stagnalis (Linn.) , which was usually the only species in nearby marsh habitats. One of the lake species is apparently undescribed (W. Moser, U.S. National Museum, personal communication, 2012); the other, H. bowermani Moser et al., 2013 , may be endemic to the lake.
Partially developed genital pores were visible on some of the largest R. klamathensis by early August, and some worms appeared sexually mature by the end of the month in 2008. Mature worms occurred as early as 20 August in 2008, occurred throughout September and October, and were present on 6 November, the last sampling date in 2008; mature worms also occurred from late August to October in other years. Cocoons were common by 8 October, and persisted through the last sampling date in 2008. A few very small worms (diameter 0.3–0.7 mm) were present in early November; these were presumably hatchlings from the 2008 reproductive season. A few of the largest worms collected in May 2008 and 2010 had faint genital pores, but internal reproductive organs were undeveloped, probably indicating resorption. Worms had no obvious reproductive structures in June-July.
Two cohorts were easily distinguished from May-July, 2008 based on the larger size and darker chloragogen of the second-year worms. The apparent first-year cohort ranged from 0.5–0.9 mm diameter in segment X in May, to 0.6–1.1 at the end of July; these smaller worms were numerically dominant in May-June. The cohorts were less distinct in August, and could not be separated by size alone in September to November, suggesting that some of the first-year worms had reached adult size. Most mature worms had darker chloragogen compared with nonreproductive worms of similar size, suggesting that R. klamathensis did not reproduce in their first year.
The Mokelumne River site is lotic, but current was very slow on the collection dates. Based on material in the grab samples, the sediment at the collection site is a deep accumulation of a silt and clay mixture. Limited material suggests a similar phenology to the Klamath population. Mature specimens were collected in October, and a single sampling effort in January retrieved only a few immature specimens.
The short reproductive period of R. klamathensis could explain its absence in prior reports (e.g., Hazel 1969; Fend & Brinkhurst 2000). Because the new species lacks a proboscis, immature specimens would not have been identified as a Rhynchelmis species using the available keys (e.g., Kathman & Brinkhurst 1998).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.