Silverstoneia flotator (Dunn)
|
publication ID |
https://doi.org/ 10.1206/3784.2 |
|
persistent identifier |
https://treatment.plazi.org/id/3F608C37-F736-FFD8-85F6-B720E302FA2A |
|
treatment provided by |
Carolina |
|
scientific name |
Silverstoneia flotator (Dunn) |
| status |
|
Silverstoneia flotator (Dunn) View in CoL
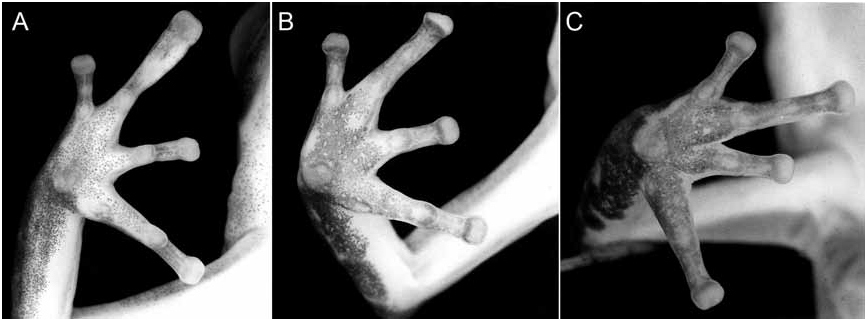
Figures 2C View FIG , 17A View FIG , 21A View FIG , 22A View FIG Phyllobates talamancae (Cope) . Part: Dunn, 1924: 5–7, pl. 2, fig. 3, 3a (tadpole). Phyllobates flotator Dunn, 1931a: 389 . Holotype lost (see text), from Barro Colorado Island, Panama.
Dunn, 1931b: 412. Phyllobates nubicola flotator Dunn, 1933: 70 ; Taylor, 1952: 647. Colostethus nubicola (Dunn) : Part: Savage, 1968: 755–757. Colostethus nubicola flotator (Dunn) : Myers and Rand, 1969: 3 (based on Ibáñez D., 1982 [thesis]). Colostethus flotator (Dunn) : Rand and Myers, 1990: 397; Ibáñez D. and Smith, 1995: 449–454, figs. 2A,
3A, 4A, 5A, 6A, 7A. Savage, 2002: 378–380, figs. 7.119a, 7.121, map 7.118, color pls. 211–212. Silverstoneia flotator (Dunn) : Grant et al., 2006: 167.
A glance at the skeletal synonymy above shows that there has been confusion as to whether Dunn’s Silverstoneia flotator is a distinct species or conspecific with his earlier named S. nubicola . Dunn named nubicola from the highlands of western Panama and the smaller flotator from the lowlands of central Panama. Later at El Valle de Antón (Dunn, 1933: 70), an intervening locality, he collected a dozen frogs with the call “of flotator ” but otherwise with characters intermediate “ between flotator and nubicola . ” Dunn concluded that “intergradation is proved” and reduced flotator to subspecific status. Savage (1968: 756) reexamined Dunn’s and other specimens from El Valle and concurred with Dunn’s interpretation. Savage discussed intrapopulational variation in nubicola but synonymized flotator .
Finally, Ibáñez D. (1982 [thesis]) showed that Dunn’s supposed intergradation between nubicola and flotator was a case of sympatry between similar species; on that basis flotator was resurrected by Rand and Myers (1990). Ibáñez D. and Smith (1995) gave published documentation for the sympatry at El Valle de Antón and Altos de Campana in west-central Panama and reviewed the taxonomy of nubicola and flotator , showing differences in vocalizations, tadpoles, coloration, and size.
Nonetheless, although Silverstoneia flotator and S. nubicola are demonstrably different species in areas of sympatry, the existence of sympatry is not universal. Furthermore, attention has been called to differences between regional populations of each species, leading to speculation that unnamed sibling species may be involved (Savage, 1968, 2002; Ibáñez D. and Smith, 1995). In any case, ontogenetic and other variation is such that individual preserved specimens often can not be unambiguously identified even within single populations of S. flotator and S. nubicola , which leads to the following discussion.
Ibáñez D. and Smith (1995) reported a number of central and western Panamanian localities for Silverstoneia flotator , with the biogeographically unlikely occurrence of an isolated population near the Colombian border, in extreme southeastern Panama (Darién). 8 They based this on a single specimen (adult male KU 115709) in a series of 13 (KU 115708–115720), of which six are adult males. While KU 115709—the purported specimen of S. flotator —is ventrally the lightest of the series of males, it is also one of the smallest. Comparison of all six males reveals continuous variation from the dark gray of this specimen to a barely darker brown in KU 115713, and finally to completely black in the largest specimen, KU 115708. Also, KU 115709 is 16.7 mm SVL, much larger than male S. flotator in central Panama, thus placing it in the size range of S. nubicola and the Costa Rican and western Panamanian populations of S. flotator . As such, we refer this frog to S. nubicola (contra Ibáñez D. and Smith, 1995; see also below).
A similar situation is observed in material assigned to Silverstoneia nubicola from 500 m in the nearby Serranía de Pirre. The small series KU 115688–115693 includes an adult male (KU 115689, the only adult male in the series) that could be considered an adult S. flotator . The throat marking is very faint and the frog is only 15.4 mm SVL, typical for S. flotator and slightly smaller than undisputed S. nubicola (KU 115706 and 115721, the next-smallest adult males, were measured at 15.5 and 15.8 mm SVL, respectively). 9 However, the largest female (KU 115688) in the series is a juvenile of 15.6 mm SVL, which is slightly larger than any juvenile from confirmed S. flotator localities (the two largest juveniles from central Panama, KU 172617 and 172690, are 15.4 and 14.8 mm SVL, respectively; the largest juveniles from the western populations are LACM 145685 and 145716, both 15.5 mm SVL). Furthermore, three additional frogs were collected at 900 m in the Serranía de Pirre, including an adult male (KU 115694) with dark, nubicola - type throat coloration and SVL of 18.6 mm, and two females (KU 115695–115696) with SVLs of 20.6 and 19.5 mm, respectively. Given that (1) in the series only one male is included from 500 m (so variation is moot), (2) in the same series the juvenile female is the size of adult S. flotator , and (3) material from 900 m on the same ridge is clearly not S. flotator , we tentatively refer these frogs to S. nubicola . Note that, although we are convinced that these populations are not S. flotator , we are not certain that they are S. nubicola (see below).
Similarly, there is evidence to suggest that the Costa Rican and western Panamanian populations currently referred to Silverstoneia flotator may not be conspecific with the central Panamanian frogs. In most specimens from the Atlantic side of western Panama (Bocas del Toro) and eastern Costa Rica (Limón) the oblique lateral stripe is short, extending from the groin to just posterior to the shoulder (e.g., see Savage, 2002: pl., 212, specimen from Atlantic western Panama), and/or dorsolateral stripes are present. However, although we did not see this color pattern in S. flotator from other regions, it is not found in all specimens at any of these Atlantic localities
8 This Darién locality is discussed and mapped under “Jaqué–Imamadó Divide” in Myers (1969: 4, 18–19, figs. 1 [locality 2], 10–11). Myers collected Silverstoneia specimens of the nubicola - flotator complex throughout Darién, in foothills and low mountains at elevations of 50–1000 m (e.g., map localities 1–4 in Myers, 1969: fig. 1). Most of these specimens have been confirmed as S. nubicola (sense lato) in Ibáñez D. and Smith (1995) or in the present study. The eastern limit of the range of S. flotator is yet to be determined, but there seems no convincing evidence that it extends anywhere close to the Colombian border.
9 We are well aware of the inherent problems of measuring error, especially when interpreting minuscule differences among small pliable animals. Nearly all measurements in this paper, including all measurements in these and similar comparisons, were made by a single person (T.G.) in order to increase reliability.
(e.g., present in KU 94702 but not KU 94687, both from the same locality in Bocas del Toro). Given that Ibáñez D. and Smith (1995: 451) found significant differences in size among samples from western Panama and central Panama, we examined SVL to see if it would lend support to the recognition of more than one species. We divided western localities into Atlantic (Bocas del Toro, Panama + Limón, Costa Rica) and Pacific (San José and Puntarenas, Costa Rica) samples and examined differences in SVL between them and the central Panamanian sample (table 3). Analysis of variance ( ANOVA; table 4) indicates that both the Atlantic and Pacific frogs have significantly greater SVL than those of central Panama. Among the western samples, the Pacific females are significantly larger than the Atlantic females, but males of these two regions cannot be distinguished on the basis of SVL (although this may be due to the smaller sample size). In conclusion, although regional variation in color pattern and SVL suggests the existence of as many as three species, available data are unable to consistently diagnose them; as such, we tentatively refer them all to S. flotator sensu lato pending additional evidence .
ON THE HOLOTYPE OF SILVERSTONEIA FLOTATOR: In addressing the taxonomy of Silverstoneia flotator , workers should be aware of a problem regarding the holotype of this taxon. Dunn (1931a) based his description of S. flotator on three specimens. He did not provide field or museum numbers for any of them. However, he did state that “Any types now in my own collection will be deposited in the Museum of Comparative Zoölogy” (p. 385) and went on to specify that the holotype is an “Adult male, in my own collection, taken July, 1930,” measuring 17 mm “head to snout [presumably snout to vent],” from “Barro Colorado Island, Panama Canal Zone” (p. 389). In his description, he explicitly stated that the holotype had the “third finger swollen” (see fig. 17A), and under Variation he stated that “A female [paratype] from the same locality is similar save for the third finger not being swollen.” The sex of the third paratype is not stated, but its locality is given as “Cana in Darien.”
Inasmuch as he was obviously aware that the third finger character is sexually dimorphic— and he explicitly describes it as being swollen—it is unlikely that Dunn would have erred in determining the sex of the holotype. Yet MCZ 16006, the specimen cataloged and known as the holotype for nearly 70 years (Barbour and Loveridge, 1946), is an adult female of 16.0 mm SVL whose third finger is not swollen. As such, we conclude that MCZ 16006 is not the holotype .
In a subsequent section of the same paper, Dunn (1931a: 392) provides a list of localities and 23 museum numbers for specimens of Silverstoneia flotator , some of which presumably correspond to the material used in his description. Much of the material is from other regions of Panama and Costa Rica and can thus be dismissed. MCZ 16006 (the supposed holotype) is never mentioned (although MCZ 16007, from Punta Bruja, is, so MCZ 16006 would already have been cataloged at the time of publication) . USNM 50177 View Materials is from “Cana, Panama,” so it is presumably the Cana specimen from the description .
Thirteen specimens are from Barro Colorado Island. Of these, five are MCZ specimens: MCZ 10728 and 15289–15292. MCZ 10728 is no longer at MCZ, and its card has a note in Loveridge’s hand that reads “Taylor” (J. Cadle, personal commun.), presumably meaning that the specimen was sent to Edward Taylor at KU. The specimen is not in the KU collection (J. Simmons, personal commun.) and may have been retained in Taylor’s personal collection, which he eventually sold to the Field Museum. Regardless, Barbour and Loveridge (1946: 171) list this specimen as having been obtained by Barbour and received by the MCZ in 1923, whereas the holotype was collected by Dunn in 1930. Of the series MCZ 15289–15292 View Materials , only 15289 and 15290 remain at MCZ. MCZ 15289 is an undissected female (finger III not swollen, vocal slits absent, 15.2 mm SVL); the slightly crushed MCZ 15290 is an adult male (finger III swollen, vocal slits present), so could conceivably be Dunn’s holotype. But it is only 14.5 mm SVL, much smaller than the 17 mm reported by Dunn. Although males of 17 mm are not known in central Panamanian populations (greatest SVL = 16.0 mm; table 1), a discrepancy of 2.5 mm seems unlikely .
In conclusion, none of the specimens currently housed at MCZ matches Dunn’s (1931a) description of the holotype. Although our search has not been exhaustive, it is sufficient to cause us to consider the holotype of Silverstoneia flotator to be lost. Insofar as no name-bearing type is necessary to define S. flotator objectively (no other species of Silverstoneia occurs at the type locality of Barro Colorado Island [Rand and Myers, 1990] and ample topotypic material resides in numerous collections), there is no need to designate a neotype ( ICZN, 1999: Art. 75).
| MCZ |
Museum of Comparative Zoology |
| KU |
Biodiversity Institute, University of Kansas |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |