Crassostrea gasar identification
|
publication ID |
https://doi.org/ 10.1016/j.jcz.2023.06.002 |
|
persistent identifier |
https://treatment.plazi.org/id/03BE87EB-FFE8-6272-FFE2-FCD5FC80FAF8 |
|
treatment provided by |
Felipe |
|
scientific name |
Crassostrea gasar identification |
| status |
|
4.2. Crassostrea gasar identification
4.2.1. Historical and taxonomic review
The first report of Crassostrea gasar appeared in Adanson’ s travel report of Senegal, in which he described oysters affixed to mangrove roots, calling them Ostreum gasar ( Adanson, 1757) . The entity itself has a clear definition of morphological traits, a complete description of conchological and anatomical characters, and habitat circumscription, and states the ethnological importance among native populations ( Adanson, 1757). However, this entity is considered an unaccepted name for designating oyster species owing to the pre-Linnean data of its publication (Art. no 3.2 of ICZN, 1999).
Gmelin (1791) then included the Ostreum gasar identity as part β among the complex Ostrea parasitica Gmelin (1791) . Gmelin also included information about O. parasitica Chemnitz (1786) , to which he attributed part γ of O. parasitica , as well as other Ostrea specimens not included in any variety, such as Rumphius (1705), Petiver (1713), Klein (1753), and Ostrea arborea Chemnitz (1785) , combining mangrove oyster specimens to a single taxon.
O. parasitica View in CoL emerged as concept to unite oyster specimens with a preference for attachment in roots of mangrove trees (especially Rhizophora sensu Linneaus (1754, p.442)) View in CoL , as Gmelin also detailed: “arborum littoralium paefertium mangiferae radicibus affixa, forma et magnitude varians”. Diverse oyster names united in O. parasitica View in CoL can be seen as an attempt to organize mangrove oyster taxa described in the first half century of the Linnean standardization of species. This conformation of O. parasitica View in CoL , bearing multiple entities, sparked later discussions regarding their lack of morphological and distributional cohesion.
For many French testaceological writers of the early 1800s, Ostrea parasitica View in CoL presented a confused synthesis, recognized as an oyster from Senegal vernacularly called “Huître Gasar” ( Blainville, 1821; Bosc, 1802; Clerc, 1828; Pasquier, 1818), in a clear recognition of the entity described by Adanson, but with distribution from the Atlantic to the Indo-Pacific Ocean, according to information proposed by Gmelin.
Dillwyn (1817), in an attempt to elucidate the oyster species described in Gmelin’ s edition of Systema Naturae, rearranged Ostrea parasitica as a synonym of O. arborea and attributed the conception of Adanson’ s Ostreum gasar (represented by Adanson (1757, pp. 196–200, t.14–1) and the copied illustration found in Brugui`ere (1792, t. 178,
Figs. 1–2 View Fig View Fig )) to a new described variety: O. arborea var. gasar Dillwyn (1817) . This must be considered the oldest nomenclatural arrangement for Adanson’ s mangrove oyster species and must not be interpreted as an entity equal to O. arborea nom . dubitum (see details in the historical and taxonomic review of C. rhizophorae ). Having elevated the entity to a category of species and rearranged its genus, Crassostrea gasar Dillwyn (1817) is the correct name and author for species representation, as it is older than the formal appearance of O. gasar Dautzenberg (1891) , O. brasiliana Lamarck (1819) or O. tulipa Lamarck (1819) .
However, further studies kept Adanson’ s species amidst Gmelin’ Ostrea parasitica since the reason for using Dillwyn’ s O. arborea var. gasar was cast aside. The acknowledgement of Ostrea gasar as a distinct species from the conceptual O. parasitica was indeed verified, although no other name was suggested for it ( Rochebrune, 1904). In fact, previous authors have given too much credence to Ostrea parasitica ’s habit, which has led them to place all oysters that attach themselves to the roots of mangroves under the same specific name, thus confusing several different species ( Deshayes and Milne-Edwards, 1836). As a result, differing views arose, including the discreet disappearance of Ostrea gasar ’s distribution status from O. parasitica ( Lamarck, 1819) to the redefinition of Adanson’ s specimen as having rounder shells, whereas O. parasitica is linguliform ( Deshayes, 1831), according to Dillwyn’ s (1817) interpretation of the species. The former use of O. gasar to address mangrove oyster species from the West African Atlantic would only appear much later in Dautzenberg (1891), which stabilized the name for this entity.
Apart from the original nomenclature incongruities, Crassostrea gasar maintained its status as a West African mangrove oyster until 2000, with a preference for low-salinity waters in equatorial estuarine areas ( Ranson, 1948).
As mangrove oyster species in the Southern Atlantic (West African and Eastern South American shores) are biologically and economically important, the taxonomic status and distribution of C. gasar , C. rhizophorae and C. brasiliana require further clarification. Based on 16S rDNA, Lap`egue et al. (2002) indicated two sets of sequences: haplotype “a” is found in West Africa and Brazil (Paranagu´a-PR, Can- an´eia-SP Bays) and French Guyana, and haplotype “b” is found in Martinique and Brazil (Paranagua´bay– PR, Salvador-BA). Since C. gasar was formerly distributed in West Africa and since some areas of Brazil have the same haplotype “a” mangrove oyster species, the results indicated a transatlantic distribution of C. gasar , questioning whether C. gasar and C. brasiliana might represent the same species.
Later, several specimens were collected along the Brazilian coast for identification of Crassostrea species in Brazil. Additionally, Brazilian and African samples from Lap`egue et al. (2002) were reevaluated. The results from the set of analyses involving 16S rDNA, COI, ITS-2 and several allozymes confirmed that C. brasiliana and C. gasar are, in fact, one species ( Lazoski et al., 2011).
The acceptance of C. gasar as the name to designate this transatlantic species started mainly in molecular studies (see Baldez et al., 2016; Cavaleiro et al., 2013; Lazoski et al., 2011; A. G. C. de Melo et al., 2010; Melo et al., 2012; Raith et al., 2015; Varela et al., 2007; Xia et al., 2014), even though C. brasiliana was a very common name still used in ecological studies to address Brazilian mangrove oyster species sympatric with C. rhizophorae . In some studies, C. gasar and C. brasiliana are maintained and discussed as likely synonyms (see Salvi et al., 2014; Salvi and Mariottini, 2017). Recently, the name C. tulipa has appeared in some South American studies (see Brunetto et al., 2020; das Chagas et al., 2019), which is an adequation to the World Register of Marine Species database. Likewise, some West African studies have historically used either C. tulipa or C. gasar to identify mangrove oyster species in this region (see Yankson, 1999, Table 1), with more recent studies applying the name C. tulipa to identify the mangrove oysters (see Chuku et al., 2020; Osei et al., 2022).
Conspecificity between C. gasar and C. tulipa was suggested in Notes sur les esp`eces Lamarckiennes d’ Ostrea ( Lamy, 1924a) based on the morphological approximation of the species. Ensuring that the name C. tulipa can be applied to any mangrove oyster species is the major concern in this study.
4.2.1.1. Are Crassostrea tulipa and C. gasar indeed conspecific?. Fundamentally, Crassostrea tulipa has been poorly described without any reference to type locality. Conchological characters were restricted to oval-oblong tortuous shells, violaceous on the right valve, and with red longitudinal lines on the left valve ( Lamarck, 1819), which can be applied to either C. gasar or C. rhizophorae in this study.
Previous studies did not attempt to establish the geographical distribution of C. tulipa , instead inferring a taxonomic approximation to Ostrea mytiloides ( Deshayes and Milne-Edwards, 1836) . Somehow, C. tulipa was also approximated to O. parasitica since Deshayes and Milne-Edwards (1836) considered O. mytiloides a smaller oyster bearing all the characters of the shell that were found in the Moluca region ( Petiver, 1713; Rumphius, 1705), in which Lamarck related to O. parasitica .
After a new interpretation of Crassostrea tulipa by Hanley (1856), this species was associated with C. rhizophorae , especially with the Honduran specimens, which have a color pattern similar to that of C. tulipa holotype, implying its distribution in the West Indies seas. Ostrea parasitica and O. arborea were also associated with Crassostrea rhizophorae in Hanley’ s catalogue, which suggested an approximation of both entities to O. tulipa . However, the first two species exhibited a wider range of distribution, as documented in their protologue. Studies have frequently mentioned O. parasitica in the Indo-Pacific seas (see Deshayes, 1831; Deshayes and Milne-Edwards, 1836; Lamarck, 1819). Although the origin of O. arborea was not indicated, it has also been attributed to Liberia-West Africa ( Büttikofer, 1885), as well as, to the Indo-Pacific in association with O. mytiloides ( Dautzenberg, 1911; Weber, 1890), or with a clear approximation to O. rivularis Gould (1851) ( Lischke, 1869).
The monography of Ostrea assumed O. tulipa as a Central American species based on information provided by Hanley (1856) on O. rhizophorae . Additionally, Ostrea aequatorialis D’ Orbigny (1847) , a species occurring in brackish waters from Peru to Ecuador (Pacific Ocean), was cautiously considered a synonym of O. tulipa , increasing the complexity of the species ( Sowerby, 1870).
In the taxonomic organization of mangrove oysters proposed by Lamy (1929a, 1924a), Ostrea parasitica sensu Sowerby (1870) , Hanley (1856) and Morch ¨(1853) and O. mytiloides were considered species located in the Indo-Pacific seas. O. arborea and O. rhizophorae were attributed to the Antilles, whereas O. tulipa was neither allied with Indo-Pacific taxa ( Deshayes and Milne-Edwards, 1836) nor with Antillean taxa, as previously suggested by Hanley (1856) and Sowerby (1870). Instead, O. tulipa was correlated with O. gasar as a mangrove oyster of the West African coast (1929a, 1924a). The author also attributed the identity of O. aequatorialis ( O. tulipa part pl. XVIII, Fig. 39 of Sowerby (1870)) to Crassostrea columbiensis Hanley (1846) , another species occurring in the Pacific American seas.
The justification of Lamy (1924a) in synonymizing O. gasar with O. tulipa was based on holotype comparison, in which oysters were attached to a tree, although Hanley (1856) also approximated O. rhizophorae to O. tulipa under the same circumstances. The lack of a striking feature for species identification, but mostly the lack of a holotype location for C. tulipa , makes the synonymization of taxa inaccurate.
Nevertheless, all taxonomic bases provided to construct a representation of O. gasar were passed inadvertently to O. tulipa . Replacement of C. gasar with C. tulipa complicates our understanding of this entity. C. gasar has a clear reference to locality, detailed description ( Adanson, 1757, species validated as Ostrea arborea var. gasar Dillwyn (1817)) as well as historical, ethnological, distributional, and commercial reports ( Gruvel, 1913; Lamy, 1922). C. tulipa , however, was considered a species with doubtful identity throughout its whole taxonomic history. Its holotype location is still unknown, as many conchologists questionably address it to different parts of the world (Mollucas in Deshayes and Milne-Edwards, 1836; Honduras in Hanley, 1856; Senegal in Lamy, 1924a; Central and South American Atlantic coasts in Sowerby, 1870), the morphological description in its prologue alludes to mostly other Crassostrea species. To date, any matter about the identity of C. tulipa has been completely based on C. gasar references.
A former mention of Crassostrea tulipa in a zoological assembly in West Africa appears to have been dated back to the last half of the 20th century by Nickl`es (1950) and Collignon et al. (1957), with a clear association with C. gasar . However, given all the problems intrinsic to the lack of cohesive information on C. tulipa identity, no consistent indication for C. rhizophorae , C. gasar , or any other species can be made. Therefore, C. tulipa should be disassociated from any reference directed to other mangrove oyster, as they represent a consistent entity.
4.2.2. Brazilian taxa ( C. brasiliana , C. paraibanensis ) and its relation to Crassostrea gasar
The two Brazilian taxa synonyms of C. gasar , whose taxonomic importance is relevant for the comprehension of the latter subtidal mangrove oysters, are C. brasiliana and C. parahibanensis .
C. brasiliana View in CoL is a small ellipsoid oyster with a yellowish right valve and whitish with purple stripes in the left valve (in the description of Lamarck, 1819) located on the coast of Brazil. It has been approximated to O. parasitica View in CoL (interpreted as an Antillean mangrove oyster taxon in von Cuvier and Voight, 1834), and it has the same color pattern as C. rhizophorae View in CoL type specimens, which latter makes identification of this species difficult.
This species has been neglected for a long period of time and is interpreted as synonym of O. parasitica ( Ihering, 1907) View in CoL or C. rhizophorae ( Lamy, 1924b) View in CoL . An alternative view of C. brasiliana View in CoL as a larger subtidal oyster started to rise in the popular book “Dictionary of animals from Brazil ( Ihering, 1940)” (see Table 3 for a review of species interpretation in Brazil), and it is subconsciously maintained on some studies, when authors considered two or more native mangrove oysters (i.e. Fairbridge, 1976; Nascimento, 1991). However, this perspective is not based on protologue or holotype data.
Most studies have opted to join all ecomorphotypes in a single mangrove oyster species in Brazil. The choice to use C. rhizophorae to represent the concept of mangrove oysters in Brazil reflected the acceptance of the historical concept of O. parasitica for the Antilles adopted by Ihering (1907), but applying the correct species name. The utilization of C. brasiliana denoted the need to establish C. rhizophorae a unique Caribbean species ( Gunter, 1951), with an application of C. brasiliana (and C. guianensis nom . nudum) for Brazilian species, especially from the states of Rio de Janeiro to Santa Catarina ( Ranson, 1967).
The separation of mangrove species in intertidal and subtidal species required a different approach. The smaller intertidal oyster, which inhabits estuarine and is most widely distributed along the Brazilian coast is identified as C. brasiliana , being C. rhizophorae its synonym ( Singarajah, 1979). The holotype of C. brasiliana has a small size and convergent shell morphology, which can be interpreted as juveniles of C. rhizophorae ( Singarajah, 1980) . The subtidal large oyster was described under the name C. parahibanensis , inhabiting the estuarine muddy bottoms of Northeast Brazil, and a possible large circle-shaped oyster near river mouths from Rio de Janeiro was suggested as a third species in Brazil ( Singarajah, 1980; 1979).
A posterior study on the mangrove oyster population found a correlation between the gradient ecology of the estuarine area and molecular data justifying their specific separation. An intertidal oyster, more adapted to saline gradient, was identified as C. rhizophorae , and the subtidal oyster allocated to hyposaline areas was identified as C. brasiliana ( Absher, 1989; Ignacio et al., 2000), establishing two mangrove oyster species for Brazil.
A conspecificity between the Brazilian taxa Crassostrea brasiliana , C. paraibanensis with West African C. gasar was confirmed through molecular analysis by Lazoski et al. (2011) and Lopes et al. (2018), justifying a union of taxa. Moreover, the addition of new sequences from C. gasar collected in Brazil, based on phylogenetic analysis involving African C. gasar sequences, corroborates the conspecificity of taxa (see Boehs et al., 2019; Galv˜ao et al., 2013).
(continued on next page)
Table 3 (continued)
Chronological Historical considerations Mangrove oyster species taxonomic from the studies recognized (currently interpretation accepted name of the species cited)
Brazil was postulated by (+ Ostrea sp. in part)
Fairbridge (1976), who studied the eating habit of
Preceramic Indians in the
Southern littoral. Based on shell remains of oysters, the author indicated two mangrove oysters:
O. arborea at the root of
Rhizophora - type substrate and the Mud or bank oyster
( O. brasiliana ), a larger oyster at the subtidal gradient. A convergence to
Ihering’ s (1940) perception on mangrove oyster species is noted. A third smaller species of rock oysters
( Ostrea View in CoL ) was speculated in the intertidal zone of open seas. Synonymizing both Singarajah (1980, 1979) C. paraibanensis View in CoL → mangrove oyster evaluated the identity of C. gasar View in CoL name and C. brasiliana View in CoL based on its C. brasiliana View in CoL → C. gasar View in CoL description of a new holotype analysis and, (+ O. arborea View in CoL , one. owing to its smaller size and C. rhizophorae View in CoL →
convergent shell C. rhizophorae View in CoL )
morphology, considered it as equal to small specimens of C. rhizophorae . In his opinion, C. brasiliana ,
C. rhizophorae and
O. arborea were synonyms,
being attributed to smaller and medium-sized specimens found in Brazil widely distributed in brackish-saline ecosystems,
while the largest estuarine oyster specimens located in the inner-lower salinity areas of the Paraiba River were identified as
C. paraibanensis . Other larger species of saline subtidal oysters from Rio de
Janeiro ( Crassostrea sp. )
appeared to be a different species from the conceptual
C. brasiliana . Unification of taxa in Harry (1985) unified all the C. virginica in part → C. virginica West Atlantic Crassostrea C. gasar taxa under C. virginica . The C. virginica in part →
author also applied the C. rhizophorae distribution of C. virginica to the Northern littoral of
Brazil, similar to that inferred by Ihering (1907;
1940). C. rhizophorae became a junior synonym of
C. virginica View in CoL , a position also verified in Abbot (1954) for the American Seashells. Genetic confirmation Absher (1989) evaluated a C. rhizophorae View in CoL of two mangrove population of mangrove C. brasiliana View in CoL → C. gasar View in CoL oysters in Brazil oysters in the estuarine areas of Parana ´and evinced two species with a certain degree of separation based on isoenzymes and environmental gradients.
Ignacio et al. (2000) also confirmed this pattern in
Table 3 (continued)
A morphological review of Crassostrea in Brazil proposed by Amaral and Simone (2014) contested the molecular data from phylogenetic studies. These authors considered the African specimens of C. gasar used in phylogenetic studies to be unreliable data to confirm the approximation between C. gasar and C. brasiliana , and they establish that both species were separated. The authors considered the Brazilian C. gasar specimens as a morphologically different entity from African C. gasar , thus ruling out conspecificity, even though similarities of DNA sequence between specimens collected in both sites were verified by Lap`egue et al. (2002) and Lazoski et al. (2011). Unfortunately, in their revision, Amaral and Simone (2014) did not include a list of African C. gasar morphologically evaluated specimen, a formal figure of C. gasar or which set of traits allows the differentiation of C. gasar from C. brasiliana .
The expected morphology of C. gasar and C. brasiliana in terms of colors, texture, or shape and colors of scars is not particularly similar, driving many Ostreidae experts to avoid union of taxa ( Huber, 2015).
Nevertheless, since genetic data still confirm conspecificity without reasonable doubts, the debate about the possible disassociation between the Southwest Atlantic and Southeast Atlantic specimens should be ignored for now.
4.2.3. Morphological and distribution review
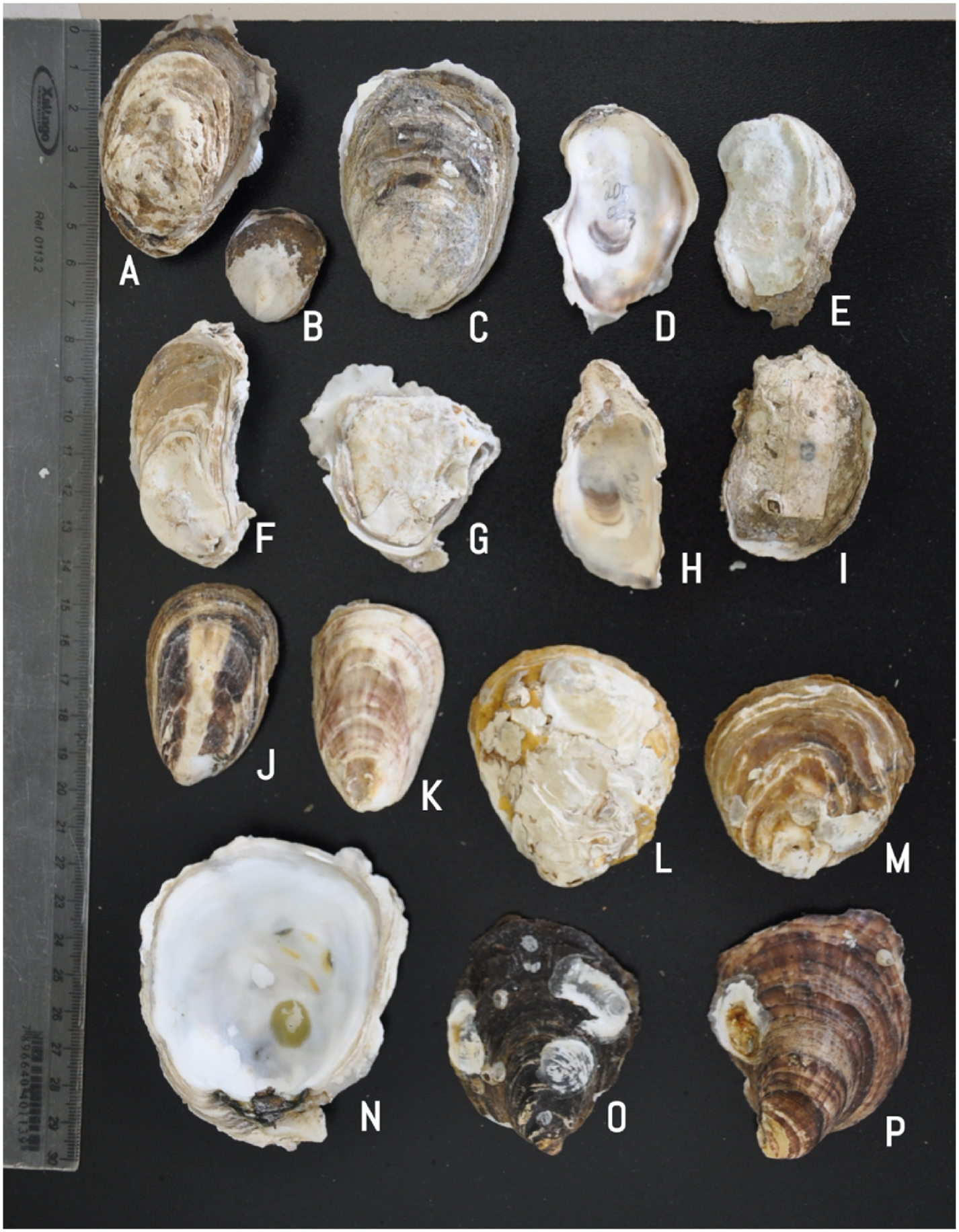
The morphological plasticity of C. gasar was greater than that in C. rhizophorae . Although morphological distinction between small- and medium-sized specimens (shell height <100 mm) is impractical for species identification, some larger ecomorphotypes have been verified for C. gasar (Supplementary material 4).
One ecomorphotype encompasses the description of C. paraibanensis , a synonym for C. gasar , which is composed of a particular set of oysters. The shell height of was greater than 100 mm, having an internal view of the right and left valves in a white coloration and the absence of an umbonal cavity, occurring solely in deep subtidal zones ( Fig. 6 View Fig ). Usually, this ecomorphotype was reported only in the Paraiba River in northeastern Brazil; however, it seems to have the same ample distribution as that of C. gasar because we have a collection of the same C. paraibanensis - like specimens in Laguna County, Santa Catarina State, the southern border distribution of C. gasar . The lack of registration of C. paraibanensis -like specimens results from the difficulty in dive sampling in high-turbidity estuaries, which is not commonly performed in surveys.
Another ecomorphotype recognized for C. gasar belongs to specimens usually collected in rocky substrates near the river mouths in more saline areas of the estuary. Usually, these oysters are so attached to the substrate, that completely removing them without damaging the integrity of the shell is a difficult job. The morphology of this particular oyster involves the greater size of SH and SL, both greater than 100 mm, with a circular SF, creating a greater approximation to Crassostrea sp. mentioned in Singarajah ( Singarajah, 1980; 1979), as well as to O. bicolor ( Hanley, 1846) .
Apart from these two morphotypes, any other aspect of the shell for C. gasar must be faced under the precepts of Crassostrea shells, in which identification is only possible with molecular data that encompasses most of the oyster population. However, the previous revision of Crassostrea gasar (= C. brasiliana ) in Amaral and Simone’ s (2014) study is mainly characterized by shells without outer undulation, deep umbonal cavity and pigmented inner surface of the left valve, occurring more frequently on rocky coasts only and in subtidal location of mangrove areas. Anatomically, these oysters would have an accessory heart in a three-branched conformation only in the right mantle lobe.
Our genetically identified C. gasar samples exhibited different shell conformations which encompassed characteristics not encountered by Amaral and Simone (2014). Some specimens had a nearly nonexistent umbonal cavity (this study, Fig. 16D View Fig ; 17 View Fig ), which is a notable variant characteristic observed between the specimens ( Carpenter and De Angelis, 2016). The syntypes of C. brasiliana also attest to the lack of a distinct umbonal cavity ( Amaral and Simone, 2014, Fig. 11E View Fig ; Singarajah, 1980, Fig. 4 View Fig ).
Furthermore, shells can have elongated muscle scar (this study,
Fig. 16H View Fig ) or outer undulations ( Fig. 15E, M View Fig ), as described by Nickl`es (1950). Some specimens had a white color at the edge of the inner surface of the left valve (this study, Fig. 15H View Fig , 16N View Fig and 17 View Fig ; Huber, 2010,
Fig. Crassostrea brasiliana ). This pattern is also described for Crassostrea paraibanensis , referring to as a “milky white and glossy” edge on the internal shell surface, as well as in the protologue of C. brasiliana ( Singarajah, 1980) , which accounts for the non-pigmented characteristic. Surprisingly, Amaral and Simone (2014) examined the holotype material of both species in question, but their description contradicts the protologues of C. paraibanensis and C. brasiliana without any discussion on the matter.
Crassostrea brasiliana View in CoL was morphologically considered a small specimen of C. rhizophorae View in CoL by most conchologists, before the existence of a molecular profile of Crassostrea species from Brazil with smaller syntypes measuring 22 × 18 mm and 18 × 15 mm ( Lamy, 1924b; Singarajah, 1980). The description of C. brasiliana View in CoL by Amaral and Simone (2014) seems to be made on oysters over 60 mm in height, which does not include the total identity of the species in question.
Our C. gasar View in CoL specimens presented shells with more morphological plasticity than C. gasar View in CoL (= C. brasiliana View in CoL ), as described by Amaral and Simone (2014). This highlights the fundamental importance of genetic identification of species prior to their morphoanatomical description and taxonomic distinction.
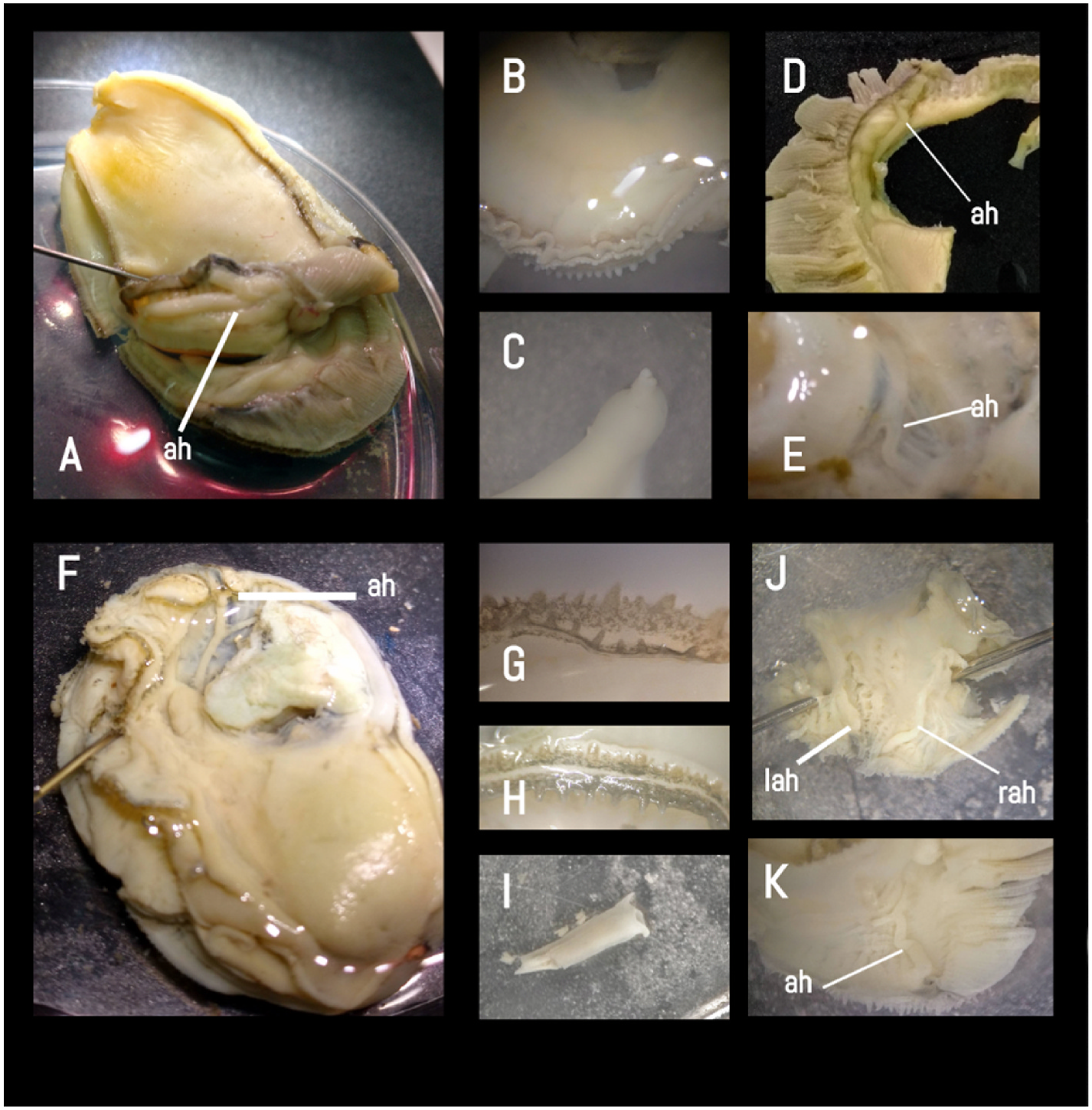
Anatomical characters are of considerable relevance in taxonomy. The mantle edges containing tentacles in C. gasar are bifurcated and usually have an outer smooth mantle projection partially covering up both rows of tentacles ( Amaral and Simone, 2014). As for the tentacle arrangement of C. gasar , our specimens had a different configuration compared to that of C. rhizophorae . Although papillae features are not considered species-specific ( Evseev et al., 1996; Harry, 1985), a few previous studies have used tentacle arrangements for species identification ( Carreon, 1969; Castillo-Rodriguez and García-Cubas, 1984). Our specimens have a tentacle arrangement with an outer mantle fold in a small juxtaposed circular projection, whereas the inner mantle fold has large finger-like tentacles spaced by the size of the tentacle ( Fig. 14B View Fig ). In contrast, C. gasar specimens from Amaral and Simone (2014, Fig. 5F View Fig and 6B View Fig ) have a tentacle arrangement with an outer mantle fold composed of long finger-like tentacles spaced from each other by a series of two to four medium or short tentacles, whereas the inner mantle fold has long finger-like tentacles spaced from each other by the size of the tentacle, which is more similar to our analyzed specimens of C. rhizophorae ( Fig. 14G View Fig ).
The description of the rectum of C. gasar by Amaral and Simone (2014) is similar to our description of C. rhizophorae ( Fig. 14I View Fig ). The rectum of C. gasar analyzed in our study ( Fig. 14C View Fig ) has a small constriction towards the anal portion, with bilabial anal folds, whereas Amaral and Simone’ s (2014, Fig. 5K View Fig ) specimens are described as having a rectum equal in thickness along the whole length with anal folds recurved downwards.
The accessory heart is commonly used for species identification ( Evseev et al., 1996) and consists of prominent vessels along the inner surfaces of the mantle folds, starting from the margin of the mantle, slightly forward of the septum that connects the two mantle lobes, and to which the posterior ends of the gills are attached ( Hopkins, 1934). Amaral and Simone (2014) described the accessory heart of C. gasar as having three branches in a “Y” configuration in the right lobe and a reduction in the left lobe. However, we observed that this trait was quite plastic among the specimens, exhibiting either a simple long branch on the left lobe ( Fig. 14D View Fig ) or with three branches of equal size, forming a ‘Y’ ( Fig. 14E View Fig ) or a ‘V’ ( Fig. 14A View Fig ) in both lobes.
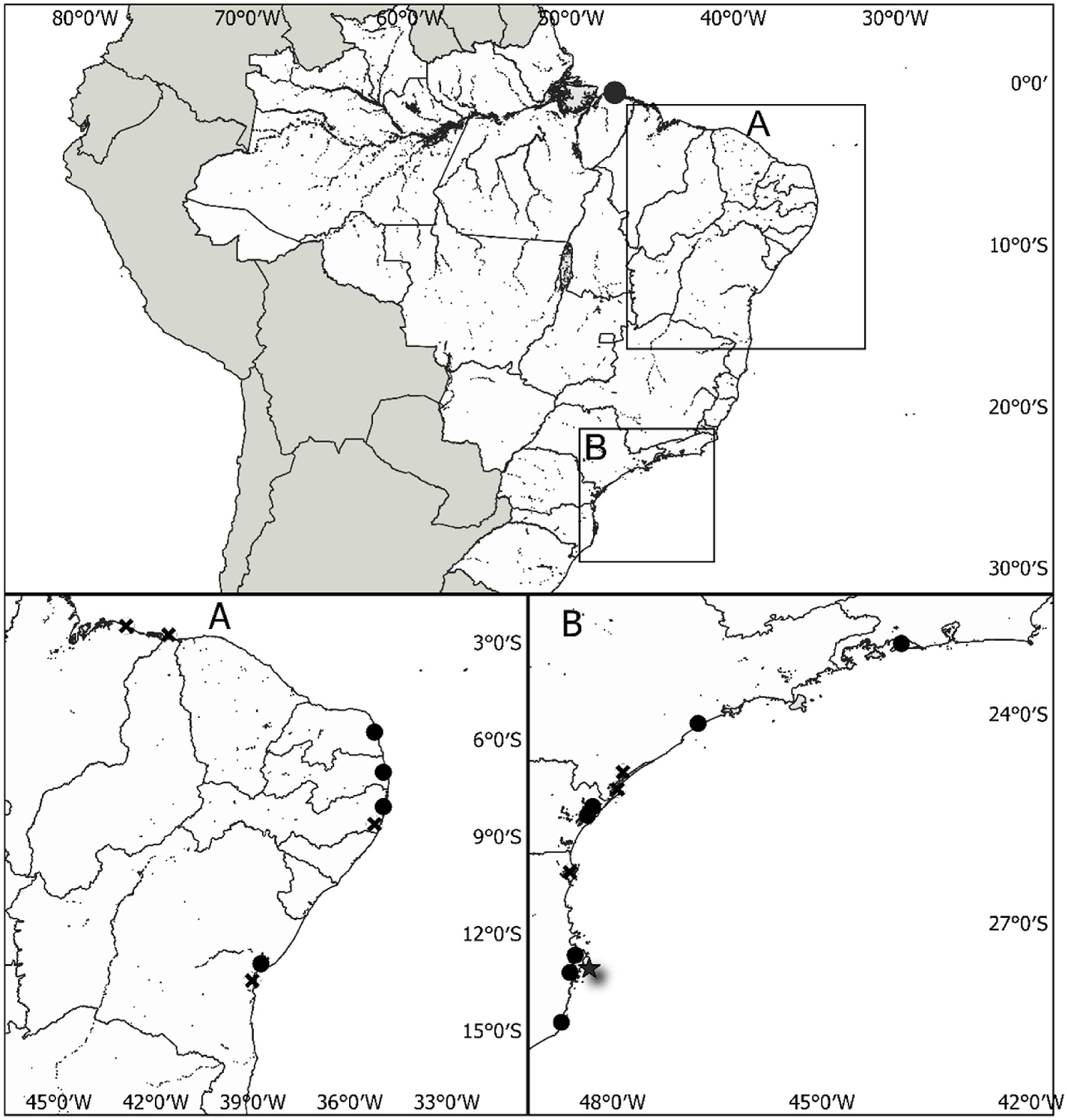
C. gasar View in CoL is distributed throughout the American Atlantic Ocean, occurring from French Guyana to Santa Catarina State, Brazil (Lap`egue et al., 2002; Lazoski et al., 2011) in mangrove roots in the intertidal zone, along with C. rhizophorae View in CoL . However, subtidal rocks and muddy substrates in less saline waters are also the predominant niches of this species ( Almeida et al., 2014; Boehs et al., 2019; Galv˜ao et al., 2013; Legat et al., 2008; Silva, 2015). C. gasar View in CoL can also be found on rocky seashores ( Amaral and Simone, 2014; Lopes et al., 2018), more or less associated with estuarine mouths. In this study, only two C. gasar View in CoL specimens were found near beach shores in the saline rock littoral area of “Praia da Barra View in CoL ” Laguna County (collected by C. M. R. Melo et al., 2010).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Crassostrea gasar identification
| Ferreira, João Paulo Ramos, Legat, Angela Puchnick, Lazoski, Cristiano, Freire, Thais Brito, Gomes, Carlos Henrique Araújo de Miranda & de Melo, Claudio Rodrigues Manoel 2023 |
C. paraibanensis
| Singarajah 1980 |
C. rhizophorae
| Rios 1970 |
C. rhizophorae
| Rios 1970 |
C. rhizophorae
| Rios 1970 |
C. rhizophorae
| Rios 1970 |
C. rhizophorae
| Rios 1970 |
C. gasar
| Vazzoler 1963 |
C. gasar
| Vazzoler 1963 |
C. gasar
| Vazzoler 1963 |
C. gasar
| Vazzoler 1963 |
C. gasar
| Vazzoler 1963 |
C. gasar
| Vazzoler 1963 |
C. gasar
| Vazzoler 1963 |
C. gasar
| Vazzoler 1963 |
C. virginica
| Gmelin sensu Dall and Simpson 1902 |
O. parasitica
| sensu Sowerby 1870 |
C. rhizophorae
| Guilding 1828 |
C. brasiliana
| Lamarck 1819 |
C. brasiliana
| Lamarck 1819 |
O. parasitica
| Chemnitz 1786 |
O. parasitica
| Chemnitz 1786 |
O. parasitica
| Chemnitz 1786 |
Ostrea parasitica
| Chemnitz 1786 |
O. arborea
| Chemnitz sensu Morch 1785 |
Rhizophora
| sensu Linneaus 1754: 442 |