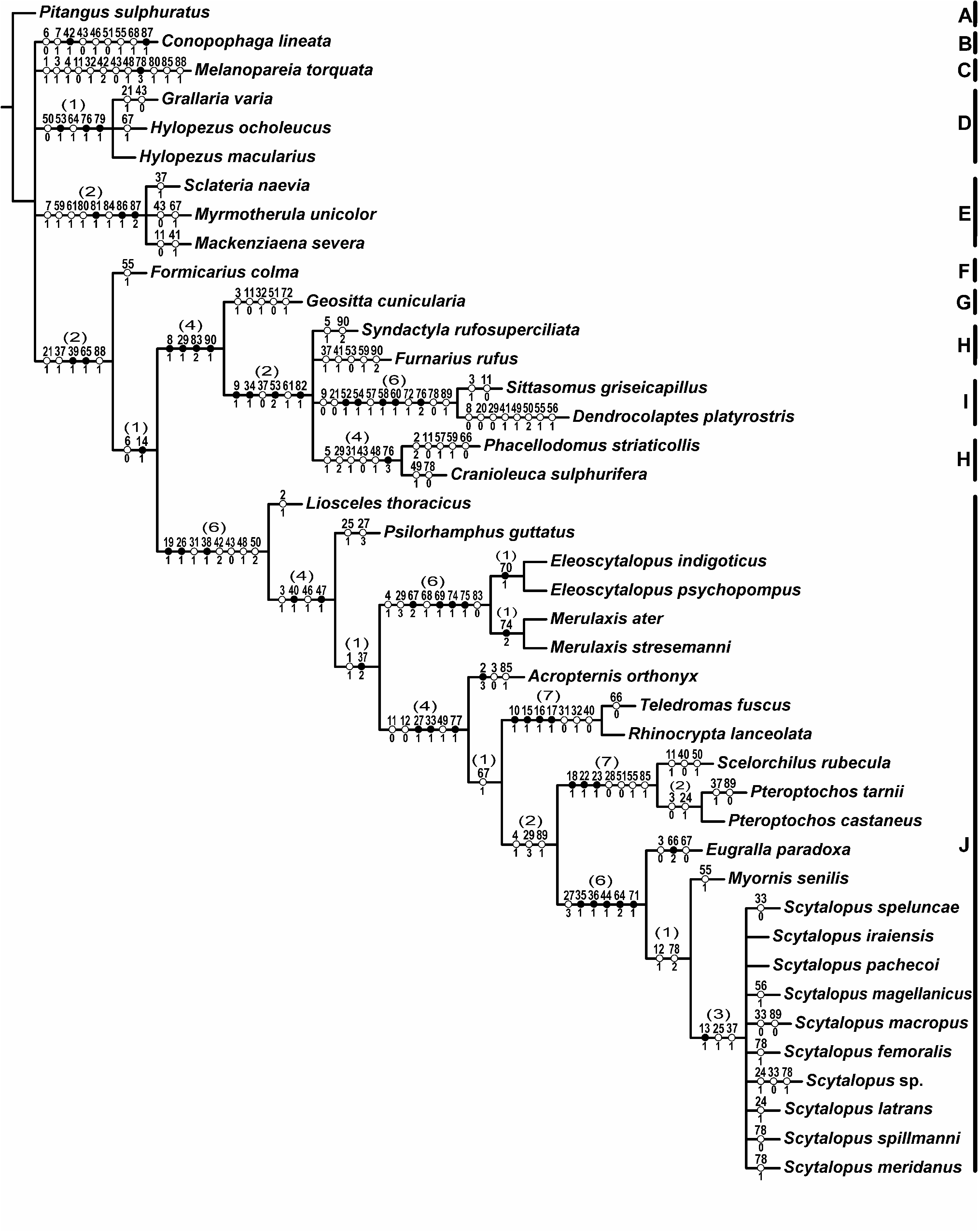
RELATIONSHIPS WITHIN
RHINOCRYPTIDAE
View in CoL
Several previous hypotheses of relationships within
Rhinocryptidae
were corroborated in the present study, but others were not. The polytypic genera
Merulaxis
,
Eleoscytalopus
,
Pteroptochos
, and
Scytalopus
were recovered as monophyletic in the analysis, but because only one of the two species of
Scelorchilus
was included here, its monophyly remains to be properly tested with the inclusion of
S. albicollis
.
The paraphyletic nature of
Scytalopus
with respect to the inclusion of the white-bellied taxa ‘S’
indigoticus
and ‘S’
psychopompus
in the genus, as shown by Maurício et al. (2008), was corroborated here, thus supporting the erection of the genus
Eleoscytalopus
to place these two species. This proposition was based primarily upon a molecular phylogenetic analysis that recovered an
Eleoscytalopus
+
Merulaxis
clade, but also included some syringeal and osteological data from an early phase of the project that resulted in the present study (see Maurício et al., 2008). The syringeal character-states suggested by these authors as support for a sister-taxon relationship between
Eleoscytalopus
and
Merulaxis
– cranial portion of the Processus vocalis with a soft consistency and A3–A5 elements dorsally reduced/absent – were optimized here together with six others (four syringeal and two osteological; see above) as synapomorphies for a clade containing exclusively these two genera, although the placement of this clade within the
Rhinocryptidae
was considerably distinct between the two studies ( Fig. 43
View Figure 43
).
A close relationship between
Scelorchilus
and
Pteroptochos
has been suggested by early taxonomists (e.g. Sclater, 1874) and was recently corroborated by molecular data ( Chesser, 1999; Maurício et al., 2008; Moyle et al., 2009; Ericson et al., 2010). In the present study this relationship was strongly supported, with seven synapomorphies, none of which has been previously mentioned as evidence of a relationship between these genera. One of these synapomorphies, presence of a dorsoventral intrinsic muscle in the syrinx, was described as having a generalized occurrence in the
Rhinocryptidae ( Ames, 1971)
, and was suggested as a synapomorphy for the family as a whole ( Rice, 2005). This hypothesis is herein refuted as this dorsally originating intrinsic muscle actually has a very restricted occurrence in the family (i.e. only in
Acropternis
,
Scelorchilus
, and
Pteroptochos
).
A
Rhinocrypta
View in CoL
+
Teledromas
View in CoL
clade was recovered by Moyle et al. (2009) and the present study, but Ericson et al. (2010) found
Teledromas
View in CoL
as sister to a
Rhinocrypta
View in CoL
+
Acropternis
View in CoL
clade. Morphological evidence in favour of the
Rhinocrypta
View in CoL
+
Teledromas
View in CoL
arrangement was solid. Besides being defined by seven synapomorphies, a general similarity in size and proportions of the sternum, cranium, and pelvis characterizes the members of the
Rhinocrypta
View in CoL
+
Teledromas
View in CoL
clade. Two of the seven synapomorphic conditions supporting this branch were previously described for
Rhinocrypta
View in CoL
only, namely parietal and frontal bones fully pneumatized ( Feduccia & Olson, 1982; Krabbe & Schulenberg, 2003) and postorbital and zygomatic processes fused ( Claramunt & Rinderknecht, 2005).
On the basis of external similarities it has been suggested that the genera
Merulaxis
View in CoL
,
Eugralla
View in CoL
,
Myornis
View in CoL
, and
Scytalopus
View in CoL
form a clade, and that within this clade
Merulaxis
View in CoL
and
Myornis
View in CoL
would be sister-taxa ( Krabbe & Schulenberg, 1997, 2003; Irestedt et al., 2002). Recent molecular studies recovered such a clade, but
Merulaxis
View in CoL
was sister to the recently described genus
Eleoscytalopus
View in CoL
instead of to
Myornis
View in CoL
( Maurício et al., 2008; Mata et al., 2009; Ericson et al., 2010). The present study partially corroborated the molecular findings, as it recovered
Eleoscytalopus
View in CoL
as sister to
Merulaxis
View in CoL
and also a well-supported
Eugralla
View in CoL
+
Myornis
View in CoL
+
Scytalopus
View in CoL
clade, but these two clades were placed in very distinct points of the rhinocryptid morphology-based tree: whereas the former is a basal branch the latter is an apical clade embeded within a clade containing the large-bodied genera. Of the six synapomorphies that define the
Eugralla
View in CoL
+
Myornis
View in CoL
+
Scytalopus
View in CoL
clade only the unfused clavicles were previously mentioned as a supporting character for this grouping ( Maurício et al., 2008; see also Feduccia & Olson, 1982). Within this clade all molecular phylogenies recovered
Eugralla
View in CoL
as sister to
Scytalopus
View in CoL
and
Myornis
View in CoL
as basal to both ( Maurício et al., 2008; Mata et al., 2009; Moyle et al., 2009; Ericson et al., 2010), thus differing from the morphological data which showed
Eugralla
View in CoL
as sister to a
Myornis
View in CoL
+
Scytalopus
View in CoL
clade. The morphological data did not allow us to test previous hypotheses of relationships in the genus
Scytalopus
View in CoL
(e.g. Arctander & Fjeldså, 1994; Krabbe & Schulenberg, 1997; Bornschein et al., 1998; Maurício, 2005; Mata et al., 2009). The strict consensus of most parsimonious trees could not even recover the phylogenetic subdivision of the genus into an Andean and a Brazilian component as proposed by Mata et al. (2009).
The present study did not corroborate the division of the family into the subfamilies
Rhinocryptinae
and Scytalopodinae as proposed by Moyle et al. (2009). The comprehensive molecular phylogeny of Ericson et al. (2010) also diverged from the results of the former authors in that the Scytalopodinae, to be a monophyletic group, should include
Scelorchilus
and
Pteroptochos
, both being part of
Rhinocryptinae
sensu Moyle et al. (2009)
, as well as
Eleoscytalopus
and
Merulaxis
, not sampled in the latter study. The genera
Liosceles
and
Psilorhamphus
formed a clade in Ericson et al. (2010) that was sister to a
Teledromas
+
Rhinocrypta
+
Acropternis
clade, thus approaching the composition of the
Rhinocryptinae
as recovered by Moyle et al. (2009) who, however, did not sample
Psilorhamphus
. In contrast to these molecular phylogenies, the basal relationships recovered by the present morphological analysis consisted of
Liosceles
and
Psilorhamphus
as being successively basal to a clade containing the remaining ten genera, within which
Eleoscytalopus
+
Merulaxis
were sister to a group composed of the remaining eight genera. Of this branching scheme, the basal position of
Liosceles
relative to the rest of the family and the grouping of eight genera were supported by four or six synapomorphies and relatively strong Bremer values (i.e. 4), and thus at least these nodes may be regarded as good topological alternatives of the deeper rhinocryptid cladogenesis relative to the molecular findings.
In summary, clades supported by six or more synapomorphies and Bremer values of 6 or 7, such as
Eleoscytalopus
+
Merulaxis
,
Scelorchilus
+
Pteroptochos
,
Rhinocrypta
+
Teledromas
, and
Eugralla
+
Myornis
+
Scytalopus
( Fig. 43
View Figure 43
), were the main points of congruence between the present morphological phylogeny and the previous phylogenetic work with the family, all sequence-based. On the other hand, more inclusive nodes (i.e. those including more than three genera) were dissimilar between this study and the molecular phylogenies, although two of those nodes received Bremer support values of 4 in the morphological analysis. None of the synapomorphies supporting these basal nodes was previously mentioned in the literature.
THE IMPORTANCE OF MORPHOLOGY IN PHYLOGENETIC INFERENCE
The differences between the results of Moyle et al. (2009) and Ericson et al. (2010) regarding the placement of some genera, in addition to the substantial topological differences found by the latter authors when the three nuclear genetic markers are analysed separately ( Ericson et al., 2010: 343, fig. 3), revives the debate concerning species trees versus gene trees: ‘If the evolution of a gene differs from that of a species, trees reconstructed from molecular data may give well-supported wrong answers to questions about species phylogeny’ ( Hillis & Wiens, 2000) and ‘There are many factors that may cause molecular analyses to reconstruct clades that are both incorrect and statistically well supported...’ ( Wiens, 2004). In this context, it is important to bear in mind that ‘a typical set of morphological characters should draw on information from many different unlinked genes [...], whereas the characters in a given molecular data set are often linked and inherited as a single unit’ ( Wiens, 2004). Therefore, as Wiens (2004: 654) states, given that we are not at a stage where all molecular phylogenies can be reconstructed without error, it is important to have rigorous morphology-based phylogenies as a ‘reality check’ for molecular results. It is also important to consider the more complex (i.e. less parsimonious) evolutionary pathway of morphological characters implied by the molecular phylogenies. For example, the phylogenetic placement of
Liosceles
both in Moyle et al. (2009) and in Ericson et al. (2010) implies reversals or parallel transformations in several characters, among which are complex ones such as the presence/absence of an osseous wall in the fossa pneumotricipitalis (character 47) and fusion of the dorsal iliac crests to form the
Crista iliosynsacralis
(character 49). Likewise, although the clade
Teledromas
+
Rhinocrypta
was supported by seven morphological synapomorphies, had a Bremer support of 7 and was recovered by Moyle et al. (2009), it was never recovered by Ericson et al. (2010). The clade (
Teledromas
(
Acropternis
+
Rhinocrypta
)) recovered by the combined analysis of Ericson et al. (2010) would imply reversals in
Acropternis
or independent evolution in
Teledromas
and
Rhinocrypta
of seven character states, including four that are exclusive to the clade formed by these two genera in the morphological tree. Here and again, we echo the claims of Wiens (2004: 654) about the importance of having rigorous morphology-based phylogenies as a ‘reality check’ for molecular results.