Gorgocephalus kyphosi, MANTER, 1966
|
publication ID |
https://doi.org/10.1093/zoolinnean/zlab002 |
|
publication LSID |
lsid:zoobank.org:pub:AAA956A8-14F7-49E4-888F-072FAC7D3826 |
|
DOI |
https://doi.org/10.5281/zenodo.5761782 |
|
persistent identifier |
https://treatment.plazi.org/id/03815953-FFA6-2E05-1490-7B8FFBC31994 |
|
treatment provided by |
Plazi |
|
scientific name |
Gorgocephalus kyphosi |
| status |
|
GORGOCEPHALUS KYPHOSI MANTER, 1966 View in CoL
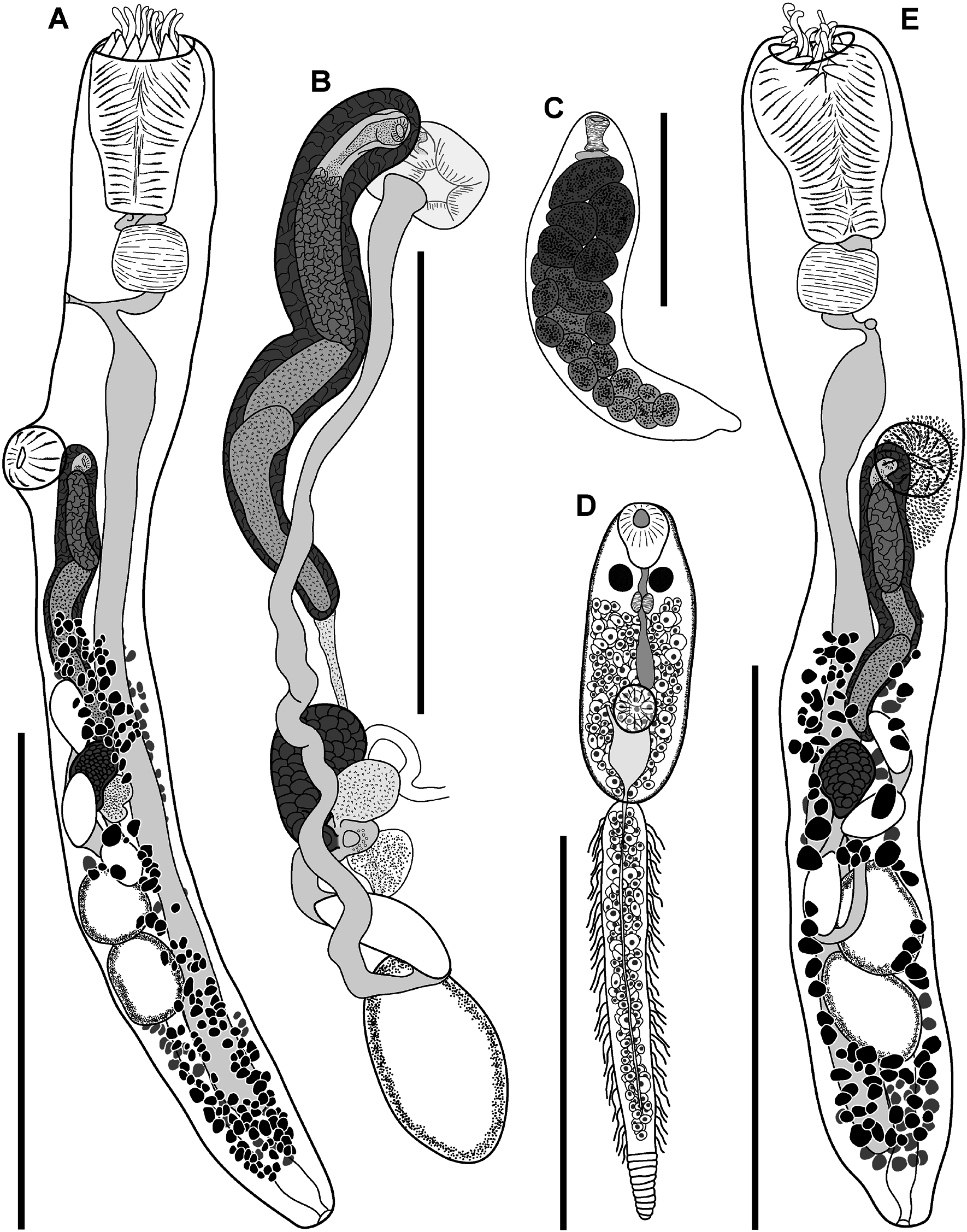
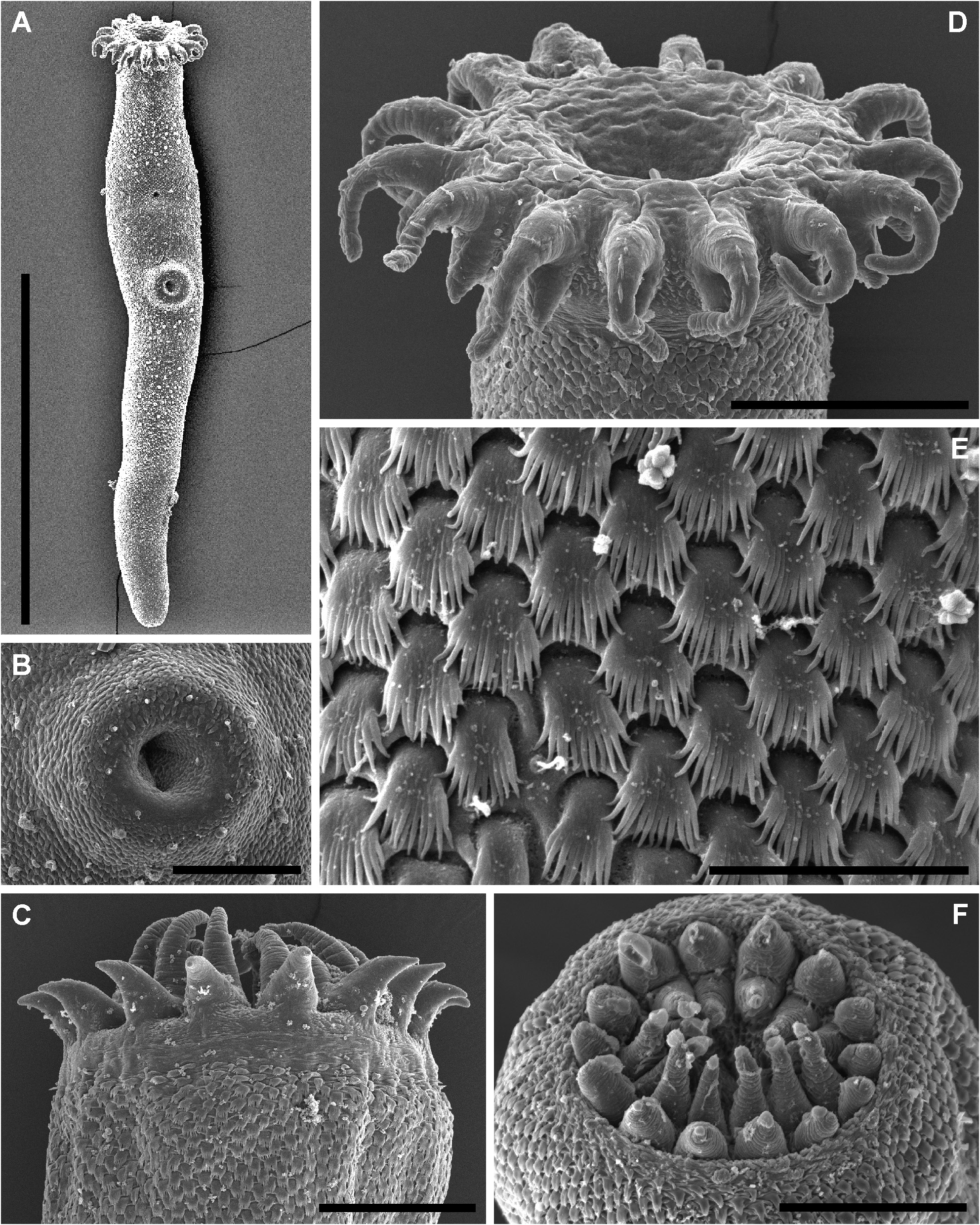
( FIGS 7–9 View Figure 7 View Figure 8 View Figure 9 ; TABLES 2 View Table 2 , 6)
Type host and locality: Kyphosus sydneyanus (Günther, 1886) ( Perciformes : Kyphosidae ), silver drummer, from near Port Noarlunga, South Australia ( 35°09’10’’S, 138°27’49’’E).
Records: 1, Manter (1966); 2, Olson et al. (2003); 3, Bray (2005b). 4, Bray & Cribb (2005); 5, present study.
Definitive hosts: Perciformes , Kyphosidae . Kyphosus cinerascens (Forsskål, 1775) , highfin chub (5); Kyphosus elegans (W. K. H. Peters, 1869) , Cortez sea chub (5); Kyphosus sydneyanus (Günther, 1886) , silver drummer (1, 5). Kyphosus vaigiensis (Quoy & Gaimard, 1825) , brassy chub (2, 3, 4, 5). The number in brackets refer to the ‘records’ section directly preceding this - essentially a way to avoid having to list citations repeatedly.
Intermediate hosts: Gastropoda, Littorinimorpha , Littorinidae . Bembicium auratum (Quoy & Gaimard, 1834) (5); Echinolittorina vidua (Gould, 1859) (5).
Other localities: Off Point Riley , Yorke Peninsula, South Australia ( 33°52’49’’S, 137°35’52’’E) (PR) (5) GoogleMaps ; off Amity Point , North Stradbroke Island, Moreton Bay, Queensland, Australia ( 27°23’53’’S, 153°26’15’’E) ( AP) (5) GoogleMaps ; off Dunwich , North Stradbroke Island, Moreton Bay, Queensland, Australia ( 27°29’46’’S, 153°23’52’’E) ( DW) (5) GoogleMaps ; off Lizard Island , Great Barrier Reef, Queensland, Australia ( 14°41’10’’S, 145°28’15’’E) (LI) (2, 3, 4, 5) GoogleMaps ; off Moorea , Society Islands, French Polynesia ( 17°32’46’’S, 149°49’47’’E) (4) GoogleMaps ; off Rangiroa , Tuamotu Islands, French Polynesia ( 15°10’40’’S, 147°39’04’’W) ( RA) (5) GoogleMaps . The letters in brackets are an abbreviation for the locality.
Voucher material (adult): Ten whole-mount and three hologenophore specimens, ex K. sydneyanus from PR (SAM AHC36801–36806); ten wholemount and three hologenophore specimens ex K. cinerascens from AP (QM G238552–G238564); two whole-mount specimens ex K. cinerascens from LI (QM G238571–G238572); 13 whole-mount and three hologenophore specimens ex K. vaigiensis from LI (QM G238573–G238588); nine whole-mount and three hologenophore specimens ex K. cinerascens from RA (MNHN HEL1431–1442); one whole-mount and three hologenophore specimens ex K. elegans from RA (MNHN HEL1433–1446).
Voucher material (intramolluscan): Six slides of rediae and cercariae ex B. auratum from DW (QM G238565– G238570); three slides of rediae and cercariae ex E. vidua from LI (QM G238589–G238591).
Site in host: Pyloric caeca (definitive); gonad/digestive gland (intermediate).
Representative DNA sequences: Twenty-one sequences deposited for COI mtDNA ( MW353629 View Materials – MW353649 View Materials ); 21 sequences deposited for 5.8S-ITS2-partial 28S rDNA ( MW353910 View Materials – MW353930 View Materials ); 12 sequences deposited for partial 28S rDNA ( MW353877 View Materials – MW353888 View Materials ); see Supporting Information, Table S2 View Table 2 .
Description of adult ( Figs 7A, B, E View Figure 7 , 8 View Figure 8 , 9A–C View Figure 9 ): Measurements in Table 2 View Table 2 . Description based on all adult voucher material plus SEM images of eight adult specimens. Body elongate, cylindrical, broadest in region anterior to ventral sucker, tapering slightly posteriorly. Tegument armed with alternating rows of partially overlapping comb-like scales; distal portion of scales forming up to 15 distinct tendrils. Eyespot pigment sparsely scattered in forebody. Oral sucker terminal, partially retractable, infundibuliform, broadest in anterior region with distinct reduction in diameter about mid-length continuing through to posterior margin; margin of anterior portion bearing crown of 14 bifid tentacles; outer branch of tentacles broad, conoid; inner branch of tentacles longer than outer branch, tendrillike, tapering distally. Ventral sucker in anterior third of body, round, far smaller than oral sucker. Prepharynx short, distinct, sigmoid or looped. Pharynx ellipsoidal to dolioform, in line with oral sucker or rotated up to 90°. Oesophagus short, bifurcates just posterior to pharynx with proximal section reaching to ventral surface and opening as ‘ventral anus’, and distal portion expanding to form caecum. Caecum single, broadest in anterior region, passes from mid-forebody to close to posterior extremity, terminates blindly; gastrodermis well developed.
Testes two, ellipsoidal, tandem, contiguous, in midhindbody. Vasa deferentia narrow, passing relatively direct from testes to cirrus-sac. Cirrus-sac elongate, cylindrical, winding, reaching from just anterior to ovary to level of ventral sucker. Internal seminal vesicle tubular, loops once about mid-length, occupies about half length of cirrus-sac. Pars prostatica distinct, vesicular, about half length of internal seminal vesicle, lined with anuclear, cell-like bodies. Ejaculatory duct short, curves back dorsally from pars prostatica to open into genital atrium. Genital atrium broad, dorsal to and larger than ventral sucker. Genital pore large, round to irregular, opening dorsally at level of ventral sucker.
Ovary pre-testicular, pyriform, narrowing posteriorly toward union with oötype. Mehlis’ gland indistinct. Laurer’s canal opens dorsally at level of ovary. Canalicular seminal vesicle saccular, contiguous with and dorsal to ovary. Uterus narrow, passes posteriorly from oötype to anterior testis, loops back, gently winding anteriorly, forming muscular metraterm about mid-level of pars prostatica, opening into genital atrium adjacent to ejaculatory duct. Eggs few, oval, operculate, large; length often exceeding that of ovary. Vitellarium follicular, restricted to hindbody; fields reaching from about mid-cirrus-sac to near posterior extremity; dorsal, lateral and ventral fields confluent, wrap around body from dorsal midline to ventrosinistral and ventrodextral regions anterior to testes, wrap entire body posterior to testes. Vitelline reservoir between ovary and anterior testis; collecting ducts indistinct. Excretory pore terminal; excretory vesicle Y-shaped, passes anteriorly, bifurcating in testicular region, ducts passing anteriorly sinistrally and dextrally, terminating as enlarged pyriform sacs on either side of cirrus-sac.
Description of redia ( Fig. 7C View Figure 7 ): Measurements in Table 6. Description based on all voucher material. Body elongate, broadest anteriorly, tapering slightly posteriorly. Cercarial embryos numerous, poorly developed. Mouth subterminal. Pharynx cylindrical to hourglass-shaped. Intestine short, saclike, immediately posterior to pharynx.
Description of cercaria ( Fig 7D View Figure 7 ): Measurements in Table 6. Description based on all voucher material. Oculate gymnocephalous cercariae. Body elongate, ellipsoidal. Eyespots two, in anterior forebody; additional pigment dispersed in forebody. Oral sucker terminal, infundibuliform. Ventral sucker post-equatorial, round. Prepharynx short, passes between eyespots. Pharynx ellipsoidal. Caecum single, terminating in region dorsal to ventral sucker. Tail longer than body, bipartite; proximal portion bearing series of lateral projections; distal portion scaled, lacking lateral projections. Excretory vesicle Y-shaped, arms extending to anterior margin of ventral sucker, stem extending posterior to ventral sucker; posterior collecting duct visible to first few scales of distal potion of tail; anterior collecting ducts not visible beyond ventral sucker. Genital primordia dorsal to ventral sucker.
Remarks: Gorgocephalus kyphosi has now been demonstrated to occur in four species of Kyphosus , the broadest definitive host range of any known member of the family. This species also occurs across a broad geographic range, from eastern and southern Australia to French Polynesia. Despite the discovery of two new species of gorgocephalid, which are morphologically similar to G. kyphosi , all previous records of this species subsequent to the type description have proven accurate. The previously available 28S rDNA sequence for G. kyphosi of Olson et al. (2003), generated from specimens from K. vaigiensis collected off Lizard Island, fell into a well-supported clade of specimens of this species from Lizard Island. Bray (2005b) and Bray & Cribb (2005) also reported G. kyphosi from Lizard Island, but again these specimens were all from K. vaigiensis ; the morphologically similar G. graboides has only been recovered from K. cinerascens . No molecular data are available to confirm the identity of specimens reported from Moorea, French Polynesia ( Bray & Cribb, 2005), but that report is again based on specimens recovered from K. vaigiensis . Off Rangiroa, Tuamotu Islands, French Polynesia, we obtained only G. kyphosi and G. yaaji , despite examination of specimens comprising three species of Kyphosus . Thus, we have little doubt that the specimens from Moorea represent G. kyphosi .
Both Manter (1966), in the original type description, and Yamaguti (1971), in study of the same material, reported the number of oral sucker tentacles of Gorgocephalus kyphosi at 14–15. We find that, because of the bifid nature of these tentacles, the fact that they are often partially retracted and that they generally overlay one-another in slide-mounted specimens, it is difficult to be confident in the accuracy of counts of these tentacles obtained during light-microscopy. This difficulty was also noted by Bray & Cribb (2005), who reported the number of oral sucker tentacles in G. yaaji as 14–17. We obtained SEM images of the oral sucker of eight individual adult G. kyphosi , including specimens from three Australian localities and French Polynesia. Although not all tentacles were visible in some of these specimens, in all of those in which an accurate count was possible, the number of tentacles was determined to be 14. Furthermore, the number of oral sucker tentacles was determined to be 14 for all species studied here, and Zhukov (1983) reported the number of tentacles in G. manteri at 14. It appears that all presently recognized species of Gorgocephalus possess 14 oral sucker tentacles.
Manter (1966) described the anterior region of the oesophagus as a ‘dorsal sac’ from which the duct opening as the ventral anus arises. Yamaguti (1971) referred to these structures collectively as the ‘post-pharyngeal vesicle’. Both of these authors also described these structures as separated from the oesophagus proper by a muscular sphincter. Bray & Cribb (2005) did not observe these features in specimens of G. yaaji or in serially sectioned specimens of G. kyphosi . We did not observe these features either, although we did not study any sectioned specimens. If such features are present, they are subtle and difficult to observe in whole-mounted specimens. Thus, we agree with Bray & Cribb (2005) that these features are unlikely to be useful for the differentiation of species.
We have discovered two intermediate hosts for Gorgocephalus kyphosi , Bembicium auratum and Echinolittorina vidua , both gastropods of the family Littorinidae . The distribution of these two gastropods suggests that G. kyphosi utilizes additional intermediate host species throughout its range. Extant species of the genus Bembicium occur only on the coasts of mainland Australia, Tasmania, Lord Howe Island and Norfolk Island ( Reid, 1988). Bembicium auratum ranges from South Australia to Lizard Island on the GBR ( Reid, 1988), covering the Australian range of G. kyphosi . Echinolittorina vidua ranges throughout the central IWP, but not to French Polynesia ( Reid, 2007). Thus, Polynesian populations of G. kyphosi must utilize an intermediate host different from those used by Australian populations, although this host will undoubtedly be a species of the Littorinidae .
No morphological differences were found between the intramolluscan stages of Gorgocephalus kyphosi obtained from Bembicium auratum and Echinolittorina vidua . The cercariae of G. kyphosi are similar to those of the family described previously ( O’Dwyer et al., 2015; Huston et al., 2016) and those of Gorgocephalus graboides described here. Although there are some subtle differences between intramolluscan gorgocephalids (see Remarks sections for G. yaaji and G. graboides ), matching of these stages to adult forms using molecular markers is likely the best approach for future work elucidating life cycles in this family.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
SubClass |
Digenea |
|
Order |
|
|
SuperFamily |
Lepocreadioidea |
|
Family |
|
|
Genus |