Brachylagus idahoensis (Merriam, 1891)
|
publication ID |
https://doi.org/10.5281/zenodo.6625539 |
|
DOI |
https://doi.org/10.5281/zenodo.6625384 |
|
persistent identifier |
https://treatment.plazi.org/id/03822308-B742-FFFF-FA17-FA0FFE4AFD60 |
|
treatment provided by |
Carolina |
|
scientific name |
Brachylagus idahoensis |
| status |
|
Pygmy Rabbit
Brachylagus idahoensis View in CoL
French: Lapin pygmée / German: Zwergkaninchen / Spanish: Conejo pigmeo
Taxonomy. Lepus idahoensis Merriam, 1891 View in CoL ,
“Pahsimeroi Valley [near Goldburg, Custer County], Idaho,” USA.
This species was formerly included in Lepus and Sylvilagus . At one time, B. idahoensis was placed with S. bachmani in Microlagus. Analysis of dental patterns showed a close relationship to Nesolagus . It has been interpreted as being either a primitive lagomorph or as derived from Sylvilagus . It is widely sympatric with Sylvilagus nuttalli and perhaps overlaps narrowly with S. audubonii . Monotypic.
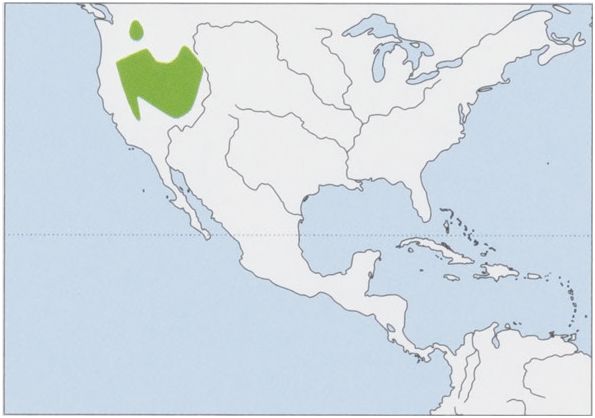
Distribution. Most of the Great Basin and some of the adjacent intermountain areas of W USA,from SE Oregon and NE California through most of C & N Nevada to S Idaho, extreme SW Montana, extreme W Wyoming, and W Utah. An isolated reintroduced population exists in WC Washington. View Figure
Descriptive notes. Head-body 230-310 mm, tail 15-24 mm, ear 56-64 mm, hindfoot 67-76 mm; weight 246-458 g. The Pygmy Rabbit is small, with short and rounded ears. Dorsalfur is buffy gray, and nape, chest, and legs are cinnamon buff. Hindlegs are relatively short, and hindfeet are broad and heavily haired. Ears are densely haired inside and outside, and their edges are buff. Tail is inconspicuous with a buff underside. The Pygmy Rabbit has one molt per year in midto late summer. New pelageis long, almost silky, and gray above, with abdomen being white. Fur changes in autumn and winter are due to wear and abrasion, and pelage appearssilver gray.
Habitat. Primarily plains and alluvial fans dominated by big sagebrush ( Artemisia tridentate, Asteraceae ) growing in tall dense stands at elevations of ¢.1370-2135 m in Nevada and ¢.1520-1615 m in California. The Pygmy Rabbit often occurs in the same habitat as the Black-tailed Jackrabbit ( Lepus californicus ) and the White-tailed Jackrabbit (L. townsendir). A high degree of vegetation cover appears to be a major habitat feature selected by the Pygmy Rabbit. Densities of big sagebrush in areas used by the Pygmy Rabbit exceed those found throughout most of the plant’s distribution. Usually, heavy grazing increases density of big sagebrush. After grazing ceases and grass cover has recovered, habitat becomes optimal for Pygmy Rabbits. This suggests that they are opportunistic, inhabiting disturbed sites in the sagebrush landscape that have an increased density of sagebrush. Intense feeding activity by Pygmy Rabbits near their burrows might sustain or even increase density of sagebrush. Pygmy Rabbits extensively use burrows that they mainly construct by themselves, so soil structure is thought to be another key habitat feature. Soft deep soils are required for burrowing. Burrows typically have 4-5 entrances but up to ten or only two have been recorded. Entrances are located at bases of sagebrush. Burrows are usually found on slopes and are oriented in a north-to-east direction. Entrance diameters are 10-12 cm. Tunnels widen below the surface, form chambers, and extend to a maximum depth of c.1 m. Pygmy Rabbits also use holes among volcanic rocks, in stone walls, around abandoned buildings, and those made by American Badgers (7axidea taxus) and Yellow-bellied Marmots (Marmola flaviventris). Dense stands of big sagebrush growing adjacent to streams, along fences, and in borrow ditches along roads might be used by dispersing Pygmy Rabbits.
Food and Feeding. Preferred diet of the Pygmy Rabbit is big sagebrush (A. tridentata); individuals sometimes even climb into tops of larger plants to feed. Big sagebrush consists up to 99% of the Pygmy Rabbit's food intake in winter. Grasses ( Agropyron spp. and Poa spp. , both Poaceae ) are more important, with 30-40% ofdiets in midto late summer. A study investigating characteristics of browsed vs. unbrowsed big sagebrush showed that higher crude protein increased chances that plants would be browsed by Pygmy Rabbits, and the opposite was the case for certain plant secondary metabolites.
Breeding. Testes of male Pygmy Rabbits increase progressively in size beginning in January, peaking in March, and regressing in June. Time of reproduction is dependent on female readiness, which is influenced by photoperiod and vegetative condition of the habitat. A study based on DNA analysis found multiple paternities in the same litter, suggesting that the Pygmy Rabbit has a promiscuous mating system. Pregnant females were collected from late February through late March in Utah and from late March through late May in Idaho. Mating behavior of captive Pygmy Rabbits consisted of chasing and brief copulations, and gestation lasted for 24 days. Females give birth in natal burrows that are separate from residential burrow systems. Natal burrows have a single entrance leading to a nest chamber. Entrances to natal burrows are backfilled with soil and often located at bases of shrubs. Female Pygmy Rabbits returned to burrows to nurse 1-2 times/day in captivity. Litter size averages 5-9 young (range 4-8 young). A maximum ofthree litters per year has been reported in Idaho. Pygmy Rabbits start breeding after their first winter of life. Adult and juvenile sex ratios were not significantly different from 1:1 in Idaho.
Activity patterns. Pygmy Rabbits are active any time within the 24hour period, but they are generally crepuscular and highly active in the morning except in winter. Activity level seems to increase at higher elevations with extreme weather conditions. Pygmy Rabbits usually rest near or inside their burrows throughout the day, but they also feed at midday. Forms under vegetation are used during daytime.
Movements, Home range and Social organization. Extensive and well-used runways of Pygmy Rabbits interlace sage thickets and provide travel and escape routes. Individuals apparently make networks of trails beneath snow at bases of big sagebrush that increase feeding areas in winter. Movementis confined to a small area (30 m radius) around the burrow in winter, with longer movements in spring and summer. Some male Pygmy Rabbits move longer distances (e.g. up to 3-5 km in two days or 1200 km in one day) during the breeding season, probably in search of females. Mean home range were 37-2 ha for females and 67-9 ha for males. Males used more burrow systems and more widely dispersed burrow systems than females. There seems to be no significant evidence of a sex bias in dispersal, but juvenile females (mean = 2-9 km, range =0-02-11-9 km) disperse farther than males (1 km, 0-03-6-5 km). Several individuals might occupy the same burrow, especially when frightened, but one adult per burrow is typical. During the reproductive season, male and female Pygmy Rabbits have been captured from the same burrow. An aggregation of 45 rabbits/ha in Idaho was reported, but it is unclear whether or notthis aggregation was due to ideal habitat or social behavior. Pygmy Rabbits make several vocalizations. They emit a loud squeal when captured as is typical of lagomorphs. An alarm call and two additional vocalizations (a squeak and a chuckle) were identified.
Status and Conservation. Classified as Least Concern on The IUCN Red List. Population status of the Pygmy Rabbitis extremely varied across its discontinuous distribution. It is locally threatened in parts ofits distribution. The Pygmy Rabbit is not ubiquitous overthis distribution but is restricted to areas of preferred habitat. A small, isolated, reintroduced population exists in Washington after the Columbia Basin population was extirpated by 2004. Habitat loss likely accounts for isolation of the Washington population. In other parts of its distribution, healthy populations remain in areas of expansive habitat. Primary threat to the Pygmy Rabbit is fragmentation of sagebrush rangeland, particularly on edges of its distribution. As these habitat patches become smaller, local extirpations of Pygmy Rabbits might occur. Probability of extirpation increases through overgrazing, uncontrolled wildfires, habitat modification, or genetic drift combined with stochastic events. Nevertheless, the fact that the Pygmy Rabbit can use sites that were once disturbed by grazing and have reverted to big sagebrush indicates that suitable habitat can be created. Where isolated populations of Pygmy Rabbits exist, corridors can be created to connect sites and thereby reduce chances of local extirpation.
Bibliography. Beauvais et al. (2008), Campbell et al. (1982), Corbet (1983), Dobler & Dixon (1990), Elias et al. (2006), Ellison (1960), Estes-Zumpf & Rachlow (2009), Estes-Zumpf et al. (2010), Falcén et al. (2011), Green (1978), Green & Flinders (1980a, 1980b), Grinnell et al. (1930), Heady & Laundré (2005), Hibbard (1963), Hoffmann & Smith (2005), Janson (1946), Johnson et al. (1950), Katzner (1994), Katzner & Parker (1997), Larrucea & Brussard (2008, 2009), Lee, J.E. et al. (2010), Nelson (1909), Orr (1940), Pritchett et al. (1987), Rachlow et al. (2005), Sanchez & Rachlow (2008), Schmalz et al. (2014), Stephenson (1966), Ulappa et al. (2014), Weiss & Verts (1984), Wilde (1978).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Brachylagus idahoensis
| Don E. Wilson, Thomas E. Lacher, Jr & Russell A. Mittermeier 2016 |
Lepus idahoensis
| Merriam 1891 |