Lepidocephalichthys eleios, Kottelat, 2017
|
publication ID |
https://doi.org/ 10.5281/zenodo.5358462 |
|
publication LSID |
lsid:zoobank.org:pub:C2F5CC5E-FA76-4357-B181-9B03D80FA398 |
|
persistent identifier |
https://treatment.plazi.org/id/36BA351B-A845-439C-AA9D-81277979D78F |
|
taxon LSID |
lsid:zoobank.org:act:36BA351B-A845-439C-AA9D-81277979D78F |
|
treatment provided by |
Valdenar |
|
scientific name |
Lepidocephalichthys eleios |
| status |
sp. nov. |
Lepidocephalichthys eleios , new species
( Figs. 2 View Fig , 3 View Fig )
Holotype. MHNG 2768.058 View Materials , 20.6 View Materials mm SL; Myanmar: Kachin State: Nam Phaung Sin Chaung (stream) near Nam Phaung Sin village, about 1.5 km before entering Lake Indawgyi north of Lonton ; 175 masl; 25°07′23″N 96°17′15″E; M. Kottelat, Nyein Chan & Dankhaung H-Hkai, 7 December 2014. GoogleMaps
Diagnosis. Lepidocephalichthys eleios is distinguished from all other species of the genus by lacking any modifications of the pectoral-fin rays in males. Pectoral-fin rays 7–8 are adjacent, with narrow or without membrane between then; in males, they are separate, not fused and have no dorsal or ventral projections. Additional characters useful to distinguish the species from congeners but not unique to it are: an irregular midlateral row of irregularly set small spots, sometimes forming a stripe; a middorsal row of 9–12 saddles, reaching downwards to about ¼ of body depth, sometimes regular, sometimes irregularly shaped and of unequal density, wider than interspaces; an arched black band at base of principal caudal-fin rays (no ocellus or black spot at caudal-fin base); caudal fin with pigments on rays and membranes forming 2–3 very irregular bars, posterior one along distal margin, bars irregularly in contact or fused, leaving variously shaped unpigmented patches; postlabial flange very long and narrow ( Fig. 4 View Fig ); head scaleless; caudal fin truncate to slightly emarginate.
Description. See Fig. 2 View Fig for general appearance and Table 1 for morphometric data of holotype and 6 paratypes. Specimens are very small, soft and difficult to handle; measurements and counts were limited to these specimens but characters were checked on all paratypes. A moderately elongate cobitid with body depth increasing posteriorly from tip of snout up to about half of predorsal distance; behind dorsal fin, body depth decreasing slowly to end of anal-fin base, then uniform until caudal-fin base. Dorsal profile continuous between head and body. Head and body very compressed. Interorbital area arched. Eye below dorsal profile of head. Snout pointed. Suborbital spine bifid, posterior (inner) prong strongly curved medially (about a quarter of a circle), length equal to about half of eye diameter, anterior (outer) prong shorter, slightly curved. Caudal peduncle depth 1.7–2.3 times in its length, of uniform depth. Largest recorded size 22.8 mm SL.
Dorsal fin with 4 unbranched and 6½ branched rays; distal margin convex; last 2 unbranched rays with space between them. Second branched ray longest. Pectoral fin with 1 unbranched and 7 branched rays; reaching about halfway to pelvic-fin base; last two branched rays (6 and 7) not fused to form a pectoral rod but adjacent, with a narrow or without membrane between them, not thickened, without dorsal or ventral projection (in both sexes). Pelvic fin with 1 unbranched and 6 branched rays; reaching about halfway to anal-fin origin; falcate; posterior margin straight; origin slightly in front of vertical through dorsal-fin origin. Anus immediately in front of anal-fin origin. Anal fin with 3 unbranched and 5½ branched rays; distal margin slightly convex. Caudal fin with 7+7 branched rays; truncate to very slightly emarginate.
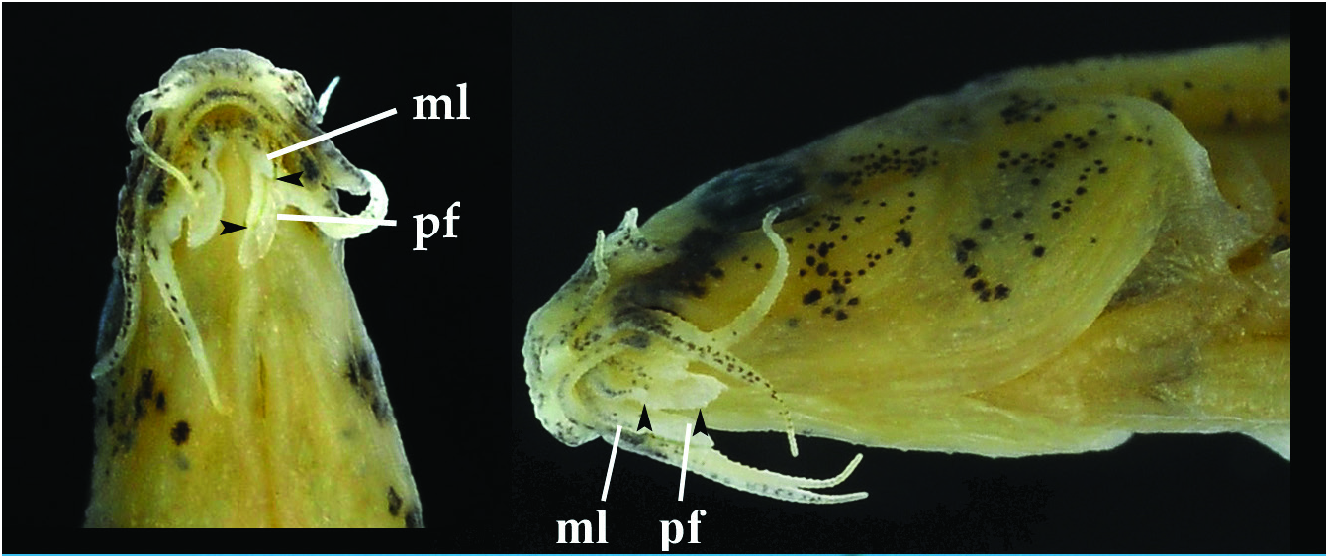
Body entirely covered by deeply embedded scales. No scales on head. No lateral line. Anterior naris at tip of an (obliquely) truncate tube; posterior naris as a long slit, adjacent to anterior one. Mouth U-shaped, gape about twice as wide as long ( Fig. 4 View Fig ). Upper lip with smooth edge. Lower lip with long mental lobe, with one or two short, thick projections along medial edge (close to base); rest of mental lobe slender and thin (hardly thicker than postlabial flange). Postlabial flange starting from tip of mental lobe, sometimes extending backwards further than tip of lobe) and with deeply concave posterior edge. Shape of flange variable. Lobe and flange sometimes together with appearance of flat ‘barbel’, especially in dehydrated specimens. Inner rostral barbel reaching beyond corner of mouth; outer one reaching beyond posterior margin of eye. Maxillary barbel reaching beyond posterior margin of eye.
Sexual dimorphism. There is no external sexual dimorphism except that in some of the largest specimens the pectoral fin is more rigid and expanded laterally ( Fig. 2 View Fig ), and in these specimens the last two branched pectoral-fin rays are adjacent, without membrane between them. These specimens are probably males: a rigid and laterally expanded pectoral fin is observed in most species of the loach families Cobitidae and Nemacheilidae and is a character of males. In the other specimens, the fins are softer, and there is a narrow membrane between the last two rays. The specimens with a narrow membrane have a somewhat stouter appearance (not quantified), as observed in females of other species of Lepidocephalichthys . See further comments under Notes on biology.
Coloration. After one month in formalin. Head and body background colour pale yellowish grey; except otherwise stated, markings darker grey to blackish. Head with stripe between eye and base of outer rostral barbel, and diffuse blotches on nape and dorsal surface of snout. Body with 9–12 middorsal saddles (3–5 predorsal, 2–3 subdorsal, 3–6 postdorsal; modally 4+2+5) reaching downwards to about ¼ of body depth, sometimes regular ( Figs. 2 View Fig , 3a, d, e View Fig ), sometimes irregularly shaped and of unequal density ( Fig. 3b View Fig ); wider than interspaces. An irregular midlateral row of irregularly set small spots or irregular blotches, sometimes forming a stripe; midlateral row or stripe leaving an unpigmented band between it and middorsal row of saddles. Otherwise, very few pigments on flank in front of level of anal fin; pigments denser posteriorly, especially on lower half of caudal peduncle. Three diffuse blotches along ventral midline: first one at anal-fin origin (on body and fin); second one at base of last anal-fin rays; third one at about midlength of caudal peduncle. Axial streak present.
Black mark at caudal-fin base: an arched band (tips pointing backwards) at base of principal rays, not reaching dorsal and ventral midlines. No ocellus.
Dorsal fin hyaline, with dark brown pigment along most of unbranched rays 3–4, most of unbranched part of branched rays; black marks on membranes near branching points. A dark spot at base of anterior rays. Caudal fin hyaline, with pigments on rays and membranes forming 2–3 irregular bars, posterior one along distal margin; bars irregularly in contact or fused, leaving variously shaped unpigment patches; pigments present on membranes (especially in posterior part of fin) and on rays, except at joints between segments. Anal fin hyaline, with dark brown pigments along most of unbranched rays 3–4, most of unbranched part of branched rays; black marks on membranes near branching points (pattern mostly visible only with magnification). A dark spot at base of anterior rays. Pelvic fin hyaline, with a few isolated dots on rays and membranes. Pectoral fin hyaline.
In life: body slightly purplish brown, slightly translucent.
Notes on biology. Lepidocephalichthys eleios was observed at 3 of 12 sites sampled in Lake Indawgyi basin: one in the outlet of the lake, two in a tributary. These three sites are characterised by deep water, very slow current, dense shore vegetation ( Figs. 5 View Fig , 6 View Fig ), large extents of floating mats of plants (Fig. 7), and bottom made of mud or clay covered with a layer of vegetal debris. The specimens were obtained from the leaf litter, from among the dense vegetation or by scraping with a tray net the underside of the ‘floating’ vegetation. Other species collected in the same micro-habitat were: Oreichthys sp. , Pethia erythromycter , Heteropneustes Remarks. The genus Lepidocephalichthys is distinguished from all other genera of the family Cobitidae by having the pectoral-fin with 1 unbranched and 7 branched rays, of which, in males, the last 2 rays are fused, forming a pectoral rod thicker than the other rays, with a variously developed projection, at about midlength of the dorsal side, ranging from a small swelling (pectoral rod swelling) to an elevated, thin, vertical flange (pectoral rod plate); in one species there is also a vertical flange along the ventral side of the ray ( Nalbant, 1963; Kottelat & Lim, 1992; Havird & Page, 2010; Deein et al., 2014). Other characters are: slender body; absence of lateral line; dorsal fin with 3–4 unbranched and 6½ branched rays; pelvic fin with 1 unbranched and 6 branched rays; anal fin with 3 unbranched and 5½ branched rays; caudal fin with 14 branched rays; three pairs of barbels (2 rostral and 1 maxillary); each half of lower lip ( Fig. 1 View Fig ) with a pointed mental lobe separated from postlabial flange by a small notch, posterior margin of flange and medial edge of lobe denticulated, crenulated or fringed; anterior naris at tip of a short tube (not extended into a ‘nasal barbel’).
fossilis, Chaudhuria sp. , Mastacembelus pantherinus , Monopterus cuchia , Indostomus paradoxus , Badis corycaeus , Dario hysginon .
Fourteen of the largest and stoutest specimens guessed to be females were dissected; either the abdominal cavity was swollen but empty, or there were unripe ovaries. Two individuals had what seemed to be one and three ova, about 0.1 mm diameter. All samples were obtained in December; a distended belly and the absence of ova suggest that spawning possibly already occured for some individuals. No specimen smaller than 15 mm SL was observed (mesh size was about 1 mm and they would have been seen). Several hours of sampling by up to 5 persons, at different depths of water and over and in different substrates yielded hundreds of individuals (of which only a subsample was preserved), but no specimen larger than 22.8 mm SL was observed. Admittedly, this does not demonstrate that they are mature, but there is also no evidence that sexual maturity is not reached at this size or smaller. The modified (rigid and curled) pectoral fins of males, the female with swollen belly (possibly spent), and the absence of small individuals suggest they are mature or close to.
Distribution. Lepidocephalichthys eleios is presently known only from Lake Indawgyi basin, Kachin State, Myanmar, in the Irrawaddy drainage. Because no sampling could be done in similar habitats downstream of the lake in the Mogaung watershed, it is premature to conclude that L. eleios is endemic to the lake and its basin.
Etymology. From the classical Greek έλΕΙΟΣ heleios, eleios, meaning ‘of the marsh’, ‘dwelling in the marsh’. A noun in apposition.
Lepidocephalichthy eleios is, however, missing the main character diagnosing Lepidocephalichthys , the presence of the pectoral rod in males. Sexual dimorphism provides very efficient characters to identify lineages in Cobitidae ( Nalbant, 1963, 1994; Šlechtová et al., 2008; Kottelat & Tan, 2008; Kottelat, 2012: 138). In specimens of L. eleios identified as males, the last two pectoral rays are not fused into a rod but close to each other, without membrane between them, they are not thickened, and there is neither a dorsal nor a ventral flange. As is described above, all the other characters used to diagnose the genus are present in L. eleios and show a similar appearance as in the other species of Lepidocephalichthys . The organisation of pectoral-fin rays 7 and 8 in close contact in male L. eleios is considered either as a plesiomorphy or as precursor stage to the fusion into a pectoral rod, and is possibly related to an incomplete development resulting from the small size of the species (developmental trunction), as observed in many other miniature fishes (see, e.g., Britz & Kottelat, 2003, 2008; Kottelat et al., 2006; Britz & Conway, 2009; Britz et al., 2014).
Lepidocephalichthys eleios cannot be a member of the other genera of Cobitidae as it does not have the characters distinguishing them from Lepidocephalichthys either. To mention only the genera present on mainland Southeast Asia: the lamina circularis in males of Cobitis and Misgurnus ; the serrated edge of the 2nd ray of the pectoral-fin in males of Kottelatlimia ; the strongly elongated body and snout and very ornamented lips with large conical and laciniated projections along the edges of Acantopsis ; the slender to anguilliform body, the backward position of the dorsal fin and the curled pectoral fin in males of Pangio ; the deep and uniformly dark brown body, small eye and poorly distinct or missing mental lobe of Lepidocephalus ; the four barbel-like projections of the lower lip and the keeled and vertically distorted caudal peduncle in males of Microcobitis ; and the ridges of papillae along the barbel and lips and the slender translucent to whitish body of Acanthopsoides .
Kottelat & Lim (1992) described sexual dimorphism in the Sundaic and some Indochinese species of Lepidocephalichthys . Havird & Page (2010) reported sexual dimorphism in most species of Lepidocephalichthys , but with a confused terminology. The main problem is their misunderstanding of the ‘lamina circularis’ present in some genera of Cobitidae . They commented (p. 137) on the fused last two pectoralfin rays: “[t]his modification is referred to as the lamina circularis and is formed by a fusion and hardening of the innermost (seventh and eighth) pectoral rays. In mature males of other cobitid genera, the lamina circularis is formed by different pectoral rays (usually the second) or is absent”. To refer to just any sexually dimorphic modification of the pectoral rays in Cobitidae as ‘lamina circularis’ is erroneous as the structures compared are not homologous (a structure on fin rays 7 and 8 vs. a structure on ray 2). There is an extensive literature on cobitids and their sexual dimorphism by authors in Europe, China, Japan, and Korea, spanning over about 140 years (e.g., Bacescu, 1962; Nalbant, 1963; Šlechtová et al., 2008). Lamina circularis means circular lamella. The sexual dimorphism in Cobitis was first described by Canestrini (1871) as a swelling at the base of the first branched pectoral-fin ray; it was later recognised as a circular, ‘scale-shaped’ projection of the ray itself and became known as Canestrini scale and later as lamina circularis, which is more appropriate because the structure is not a scale. The lamina circularis is a laminar, usually but not always circular, posterior projection of the first (proximal-most) segment of the dorsal hemitrich of the first branched pectoral-fin ray (see, e.g., Nalbant, 1963: 356; Kottelat & Freyhof, 2007: 301, fig. 61). A second lamina circularis is present on the second branched ray in some species. Obviously, the lamina circularis of males of Cobitis and Misgurnus is not homologous with the fused pectoral-fin rays 7–8 of male Lepidocephalichthys , with the thickened pectoral-fin rays 1–2 of male Theriodes , with the curled pectoral fins with thickened pectoral-fin ray 2 of male Pangio , Lepidocephalus , Acantopsis and Acanthopsoides , with the modified pectoral-fin rays 5–8 of male Neoeucirrhichthys ; or with the blade-like formation or ‘saw’ on pectoral-fin ray 2 of male Kottelatlimia ( Kottelat & Lim, 1992; Kottelat & Tan, 2008; Šlechtová et al., 2008; Kottelat, 2012; Deein et al., 2014).
Two other species of Lepidocephalichthys have been observed in Lake Indawgyi basin, L. berdmorei and L. alkaia . In both, the last 2 pectoral-fin rays are fused into a rod in adult males. Lepidocephalichthys berdmorei is a large species (up to 78 mm SL) found in small streams with moderate to fast current, usually with pebble to stone bottom. It has a stout body, yellowish brown colouration with numerous small black spots, a midlateral row of irregular small black blotches, a black blotch on base of branched caudal-fin rays 3–7; the caudal fin has 3–6 thin black bars; the last two pectoral-fin rays are fused in a thick cylindrical rod. Lepidocephalichthys alkaia reaches up to 61 mm SL and is found in more sluggish streams. It has a stout body, a pale yellowish background colour, a midlateral row of blotches more or less contiguous to form a midlateral stripe, continued by black pigments on median caudal-fin rays and membranes inbetween, a black blotch on base of branched rays 3–7 of caudal fin base, and the caudal fin has 5–8 thin irregular vertical rows of black spots; the last two pectoral-fin rays are fused into a rod with a low dorsal swelling, highest at about midlength.
Material used for comparison. Lepidocephalichthys alkaia: CMK 24128, 50; CMK 25618, 33; Myanmar: Lake Indawgyi basin. — CMK 14649, 7; Thailand: Salween drainage. L. berdmorei: CMK 24152, 2; CMK 24190, 1; Myanmar: Lake Indawgyi basin. — CMK 25430, 1; Myanmar: Tenasserim. — CMK 14648, 1; Thailand: Salween drainage. — CMK 25927, 8; CMK 25941, 3; Laos: Mekong drainage. L. cf. goalparensis: CMK 26462, 1; CMK 26518, 1; Myanmar: Irrawaddy drainage: Putao. L. cf. kranos: CMK 12646, 4; Laos: Mekong drainage. L. furcatus: CMK 16516, 5; Thailand: Tapi drainage. L. guntea: CMK 5429, 1; India: Karnataka. L. hasselti: CMK 24894, 5; Myanmar: Tenasserim. — CMK 13249, 47; Laos: Mekong drainage. — CMK 7316, 1; Indonesia: Sumatra: Siak drainage. — CMK 20580, 1; Indonesia: Sumatra: Musi drainage. — CMK 8263, 21; Malaysia: Kelantan. — CMK 5124, 24; Thailand: Sai Buri. L. jonklaasi: CMK 12190, 29; Sri Lanka. L. lorentzi: CMK 6967, 2; Indonesia: Borneo: Kapuas drainage. L. micropogon: CMK 26657, 2; Myanmar: Irrawaddy drainage: Myitkyina. — CMK 25065, 1; CMK 25230, 1; Myanmar: Tenasserim. L. thermalis: CMK 7059, 10; Sri Lanka. — CMK 9340, 12; India: Kerala. L. tomaculum: CMK 8033, 6 paratypes; Malaysia: Selangor. — CMK 11273, 7; Indonesia: Sumatra: Batang Hari. — CMK 11927, 4; Indonesia: Bintan. L. zeppelini: CMK 7968, 2; CMK 23025, 2; Laos: Mekong drainage. — CMK 10749, 5; Thailand: Mekong drainage.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |