Cnemidophorus tesselatus
|
publication ID |
https://doi.org/10.1206/0003-0082(2001)345<0001:NHBTTL>2.0.CO;2 |
|
persistent identifier |
https://treatment.plazi.org/id/03854914-4C42-FFA5-5D1C-FC74FD92B8A4 |
|
treatment provided by |
Carolina |
|
scientific name |
Cnemidophorus tesselatus |
| status |
|
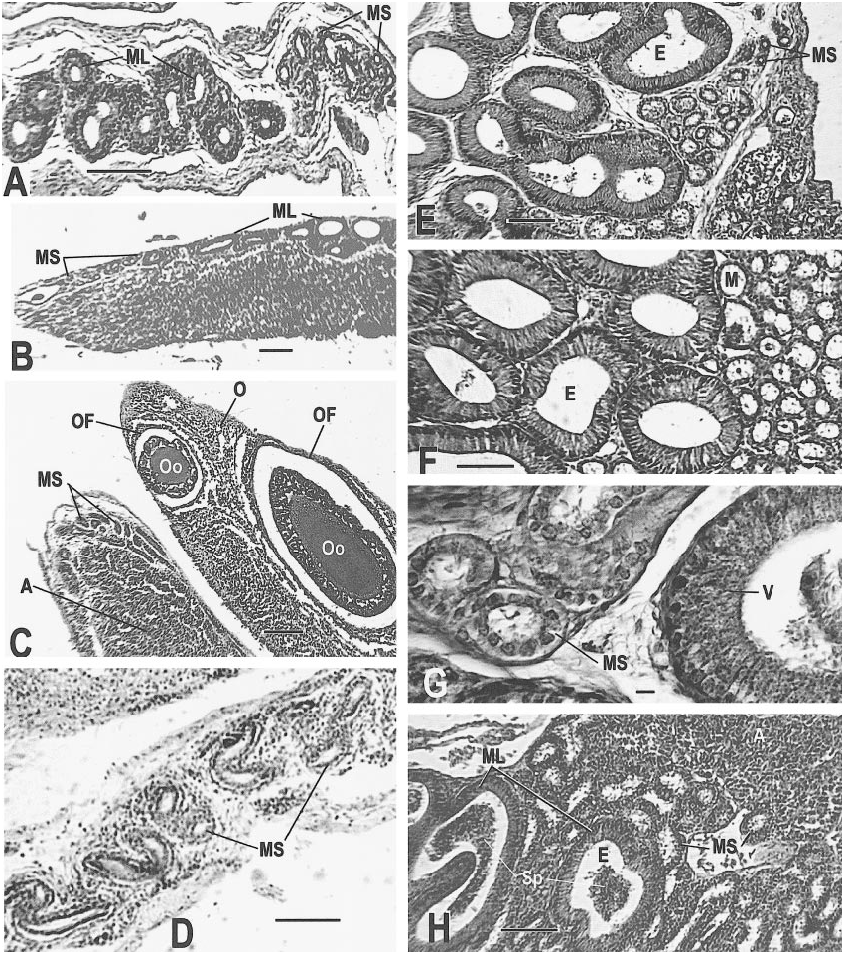
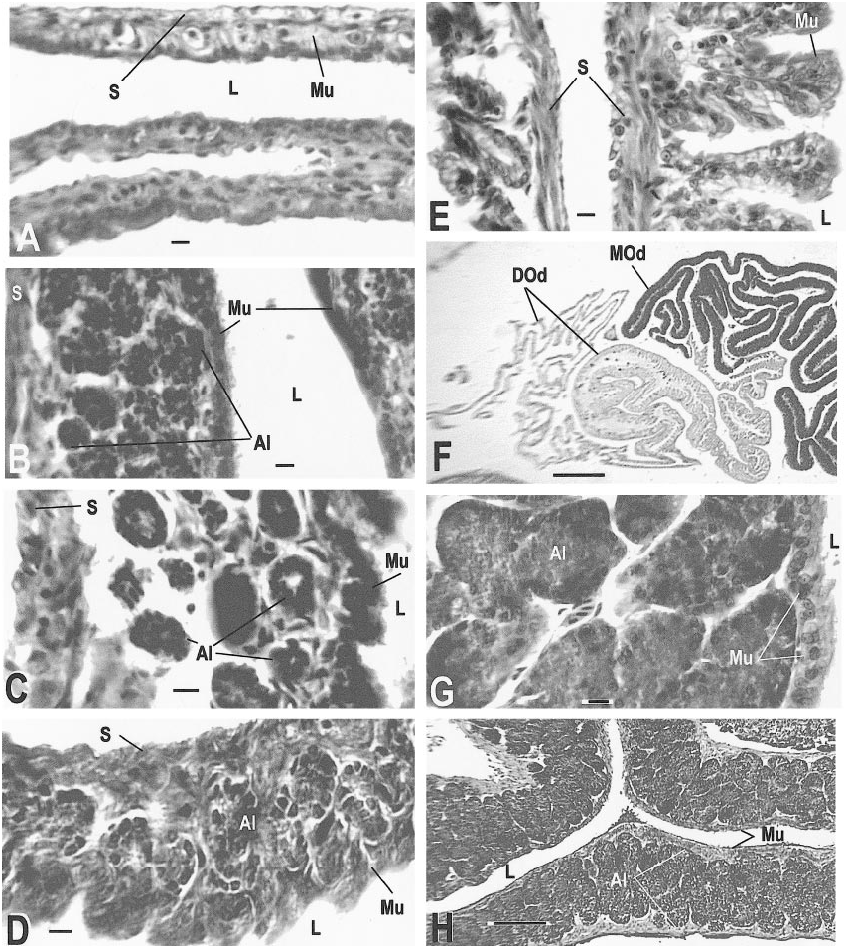
Sexually mature and reproductive adults of C. tesselatus usually exhibit the following characteristics: oocytes are present, the ovarian follicle cell layer is complete and well organized, connective tissue is not conspicuous, vacuoles are few, and the follicle is well vascularized. The distal oviduct contains a thin mucosa with poorly developed folds. The middle oviduct is ciliated and contains welldeveloped alveolar glands, which are restricted to this section of the oviduct. The proximal oviduct has a thick (normal) mucosa and welldeveloped folds. The mesonephric tubules are 20–30 µm in diameter ( figs. 15C View Fig , 18A–D View Fig ).
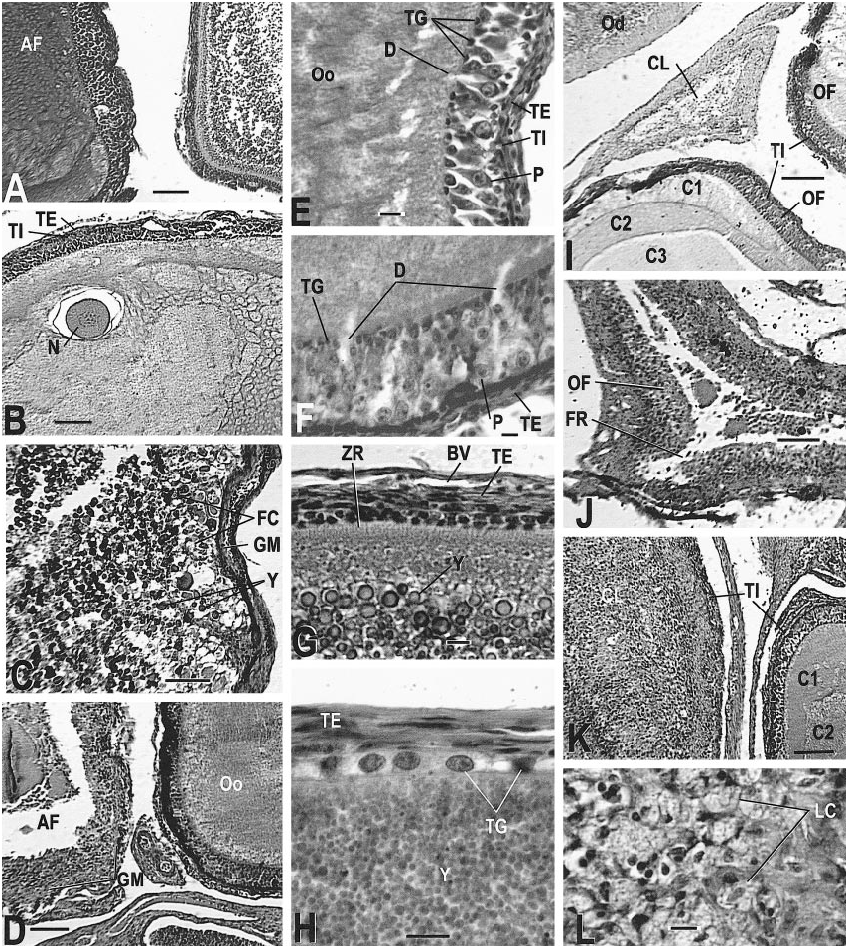
The ovary of a normal, reproductive C. tesselatus contains developing follicles with a welldeveloped tunica granulosa that includes active pyriform cells ( fig. 14E, F View Fig ). The zona radiata (formed by microvilli) is usually evident and probably indicates the active transport of components (such as phosvitin and lippovittelin) for the later syn
thesis of yolk granules ( fig. 14G View Fig ). The increase in the surface area that results from the presence of the microvilli facilitates transport of substances between the oocyte and the follicle cells (Balinsky, 1975; Anderson and Beams, 1960). The cytoplasm is granular but homogeneous in distribution in young oocytes and becomes layered in older oocytes ( fig. 14I, K View Fig ). Yolk deposition begins when the oocyte is 2.3 mm (largest nonyolked oocyte measured) to 3.1 mm (smallest yolked oocyte measured) in diameter (table 6, fig. 14G, H View Fig ); as vitellogenesis progresses the theca granulosa regresses with the loss of pyriform cells but retention of the small granulosa cells ( fig. 16G View Fig ). Atretic oocytes have stopped development for some unknown reason. A previtellogenic oocyte that is atretic shows irregular or vacuolated cytoplasm ( fig. 14A, B View Fig ). The theca granulosa is often hypertrophied, and enlarged granulosa cells move through the disrupted zona radiata or zona pellucida into the oocyte cytoplasm ( fig. 14C, D View Fig ). As atresia progresses the basement lamina of the theca granulosa becomes a hyaline layer known as the glassy membrane ( fig. 14C, D View Fig ). At ovulation the follicle wall appears thicker because the thin, stretched wall of the mature follicle is now shrunken due to the collapse of the follicle; cellular debris may be present in the lumen, but neither oocyte nor yolk will be present ( fig. 14J View Fig ). As the corpus luteum ages the lumen becomes filled with luteal cells ( fig. 14K, L View Fig ). The fully developed corpus luteum is easily distinguished from an advanced atretic follicle by the absence of yolk, absence of the glassy membrane, and the presence of vacuolated luteal cells ( fig. 14L View Fig ). Corpora lutea regress rapidly to a distinctive, triangular collapsed structure, composed primarily of connective tissue and usually located between other follicles ( fig. 14I View Fig ). Perhaps this is typical of lizard corpora lutea (Miller, 1948: pl. 13d, e for Xantusia ; Goldberg, 1970: fig. 12 for Sceloporus ).
Two individuals exhibited gonadal abnormalities. One of these (AMNH R146636), a large individual ( 95 mm snout–vent length), had very small ovaries lacking enlarged oocytes; no sperm were seen in the reproduc tive tract. The second (AMNH R146640) was the smallest C. tesselatus in the sample
(snout–vent length = 65 mm) and contained a few abnormal follicles (many irregular spaces and an incomplete theca granulosa); no sperm were found in her reproductive tract. All other representatives of C. tesselatus contained 3–22 follicles; seven individuals were undergoing vitellogenesis in follicles 1.4–8.5 mm in diameter (table 6).
Salient features of the reproductive systems of representative individuals of C. tesselatus , all C. tesselatus X C. tigris marmoratus hybrids , and all male C. tigris marmoratus collected at the hybridization site are described below with date of collection in parentheses. The first series are representatives of C. tesselatus .
AMNH R146629 ( June 11, 1998): The left ovary was 3 X 7 mm. Both oviducts were swollen; the left was 16 X 3.5 mm, the right was 14 X 4 mm. The largest oocyte had not begun vitellogenesis but the cytoplasm is differentiated into at least three zones, and the granulosa and zona radiata are well developed (as in fig. 14A, I View Fig ). Several oocytes show pyriform cells ( fig. 16H View Fig ), which indicate the onset of yolk deposition in Sceloporus (Goldberg, 1970) . At least two corpora lutea were present ( fig. 14I View Fig ). The small size and triangular shape of the corpora lutea suggest that ovulation and oviposition occurred from at least two weeks (Miller, 1948: pl. 12 for the viviparous Xantusia ) to several
months earlier (Goldberg, 1970, for the ovoviviparous Sceloporus jarrovi ); however, the sequence and timing of corpus luteum degeneration in Cnemidophorus is not well known. Two atretic follicles were present. No sperm were found.
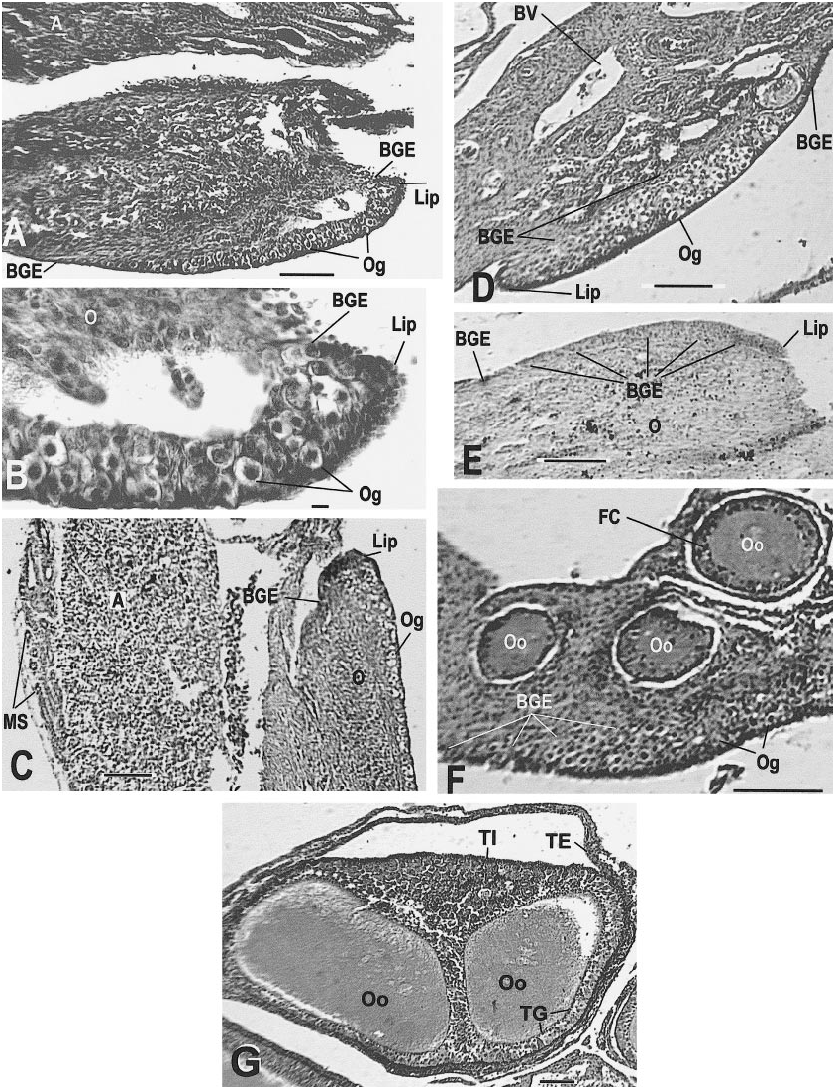
AMNH R146636 ( June 12, 1998): The left ovary and oviduct were smaller than those of the other C. tesselatus examined; however, this individual is 95 mm snout–vent length, clearly of adult size; the mean snout– vent length for the other 25 specimens examined is 89.6 ( 65–109 mm). The mesonephric kidney and adrenal were well developed and vascularized. The ovarian epithelium (cortex) was very thin and contained some oocytes and a welldeveloped lip ( fig. 15E View Fig ). The ovary consisted of a thin layer (3– 4 cells thick) over a thinner layer of smooth muscle, all of which were on the surface of the adrenal away from the mesonephros ( fig. 15E View Fig ). Follicles were not present, the ovary was probably just beyond the indifferent stage of development, and the oviduct was tiny. This animal, although of large size for a mature female, is equivalent to a hatchling in its reproductive anatomy. The karyotype and protein electrophoresis confirmed the identity of this specimen. No sperm were found .
of an oocyte; note the layered cytoplasm of the adjacent oocyte (AMNH R146629, slide 28, row 1, section 4). J. A recently ovulated follicle that will become a corpus luteum; note triangular shape and point of rupture (AMNH R146620, slide 31, row 1, section 1). K. Corpus luteum (left) and normal follicle (right; AMNH R146618, slide 1, row 2, section 13). L. Detail of corpus luteum (AMNH R 146621, slide 1, row 2, section 9). Slides stained with Mallory triple connective tissue technique are designated MT; all other slides were stained with Harris hematoxylin and eosin. Abbreviations for this and the following figures are as follows: A, adrenal gland; AC, adrenal cells; AF, atretic follicle; Al, alveolar gland; BGE, boundary of germinal epithelium; BV, blood vessel; CL, corpus luteum; CT, connective tissue; C1, outermost layer of preyolk cytoplasm of oocyte; C2, middle layer of preyolk cytoplasm of oocyte; C3, innermost layer of preyolk cytoplasm of oocyte; D, discharging pyriform cell; Dod, distal oviduct; E, epididymis; FC, follicle cell; FR, site of follicle rupture due to ovulation; GM, glassy membrane of an atretic follicle; Gr, granulosa cells of follicle; I, interrenal cells; L, lumen; LC, luteal cells; Le, Leydig cell layer; Lip, lip of germinal epithelium; LRV, left renal vein; M, mesonephros; ML, mesonephros, large tubules; Mod, middle oviduct; MS, mesonephros, small tubules; Mu, mucosa; Mus, muscularis; N, nucleus; O, ovary; Od, oviduct; OF, ovarian follicle; Og, oogonia of germinal epithelium; Oo, oocyte; P, pyriform cell in theca granulosa; S, serosa; Sp, spermatozoa; ST, seminiferous tubules; TE, theca externa of ovarian follicle; TG, theca granulosa of ovarian follicle; TI, theca interna of ovarian follicle; V, vas deferens; Y, yolk of oocyte; ZR, zona radiata. Scale bar, 0.1 mm, except E– H and L, which are 0.01 mm.
Female C. tesselatus X C. tigris marmoratus Hybrids
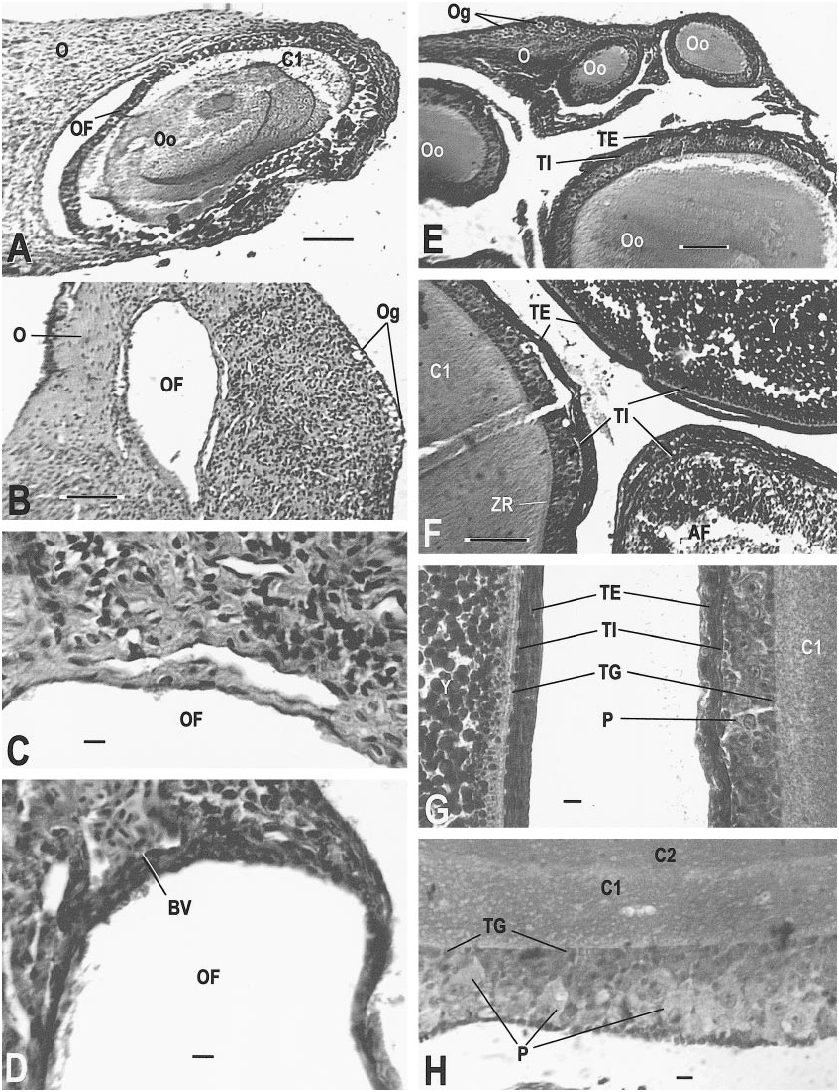
row 1, section 5). E. Germinal epithelium with lip (AMNH R146636, slide 11, row 1, section 3). F. Germinal epithelium with developing oocytes; section did not pass through lip (AMNH R146618, slide 1, row 1, section 15). G. One follicle containing two oocytes. The theca interna completely separates the two oocytes and a single theca externa encompasses the follicle (AMNH R146618, slide 4, row 2, section 2). Scale bar, 0.1 mm, except B, which is 0.01 mm.
AMNH R146637 ( June 12, 1998): The left oviduct was enlarged but lacked eggs .
The left ovary contained 14 enlarged follicles ( fig. 14F View Fig ) up to 1.4 mm in diameter and one corpus luteum. Macroscopically, the ovary showed one reddish spot, possibly the corpus luteum detected later by histological examination. The germinal epithelium contained oogonia and a distinctive lip ( fig. 15D View Fig ). The two largest oocytes had not begun vitellogenesis. No sperm were found.
AMNH R146638 ( June 12, 1998): The left ovary and oviduct were enlarged and the largest oocyte was yolked. No other oocytes were yolked and no recognizable corpora lutea were visible. No sperm were present in the oviduct .
AMNH R146639 ( June 12, 1998): The left ovary and oviduct were enlarged and the ovary contained three enlarged and yolked oocytes. The largest nonyolked oocyte was 1.9 mm in diameter, almost as large as those containing yolk, and larger by 0.5 mm than a yolked oocyte in AMNH R146637. There were at least four corpora lutea present, all flattened or triangular, and up to 1.4 mm wide. No sperm were found.
AMNH R145142 ( May 12, 1999): Two oocytes 8.5 mm in diameter were depositing yolk when preserved. Several previtellogenic follicles exhibited secretory activity of the theca granulosa and conspicuous pyriform cells ( fig. 14E View Fig ) similar to those seen in Ctenosaura (del Carmen et al., 1996). Near the end of vitellogenesis, the granulosa was still evident as a distinctive cell layer ( fig. 14H View Fig ). The distal oviduct had a very thin mucosa and very thin folds near the infundibulum, but near the junction with the middle oviduct the mucosa was thickened ( fig. 17F View Fig ). The middle oviduct contained normal alveolar glands ( fig. 17G, H View Fig ). No sperm were found .
AMNH R145143 ( May 14, 1999): This specimen had an early atretic follicle with disorganized cytoplasm ( fig. 14A, B View Fig ) and follicles that were undergoing vitellogenesis
( fig. 14G View Fig ). The oviducts were enlarged; the folds of the distal oviduct were shallow near er the infundibulum ( fig. 17E View Fig , left) and deep er away from the infundibulum ( fig. 17E View Fig , right). No sperm were found.
AMNH R146612 ( June 10, 1996): This specimen contained a yolked follicle (6.4 X 6.7 mm) and a corpus lutuem ( 0.5 mm diam.). The mesonephric tubules were small ( 0.02 mm diam.). Other ovarian and oviductal structures appeared normal. No sperm were found .
AMNH R146613 ( June 10, 1996): The ovary contained follicles of several sizes (as in fig. 16E View Fig ), including yolked and atretic follicles ( fig. 16F View Fig ). No sperm were found .
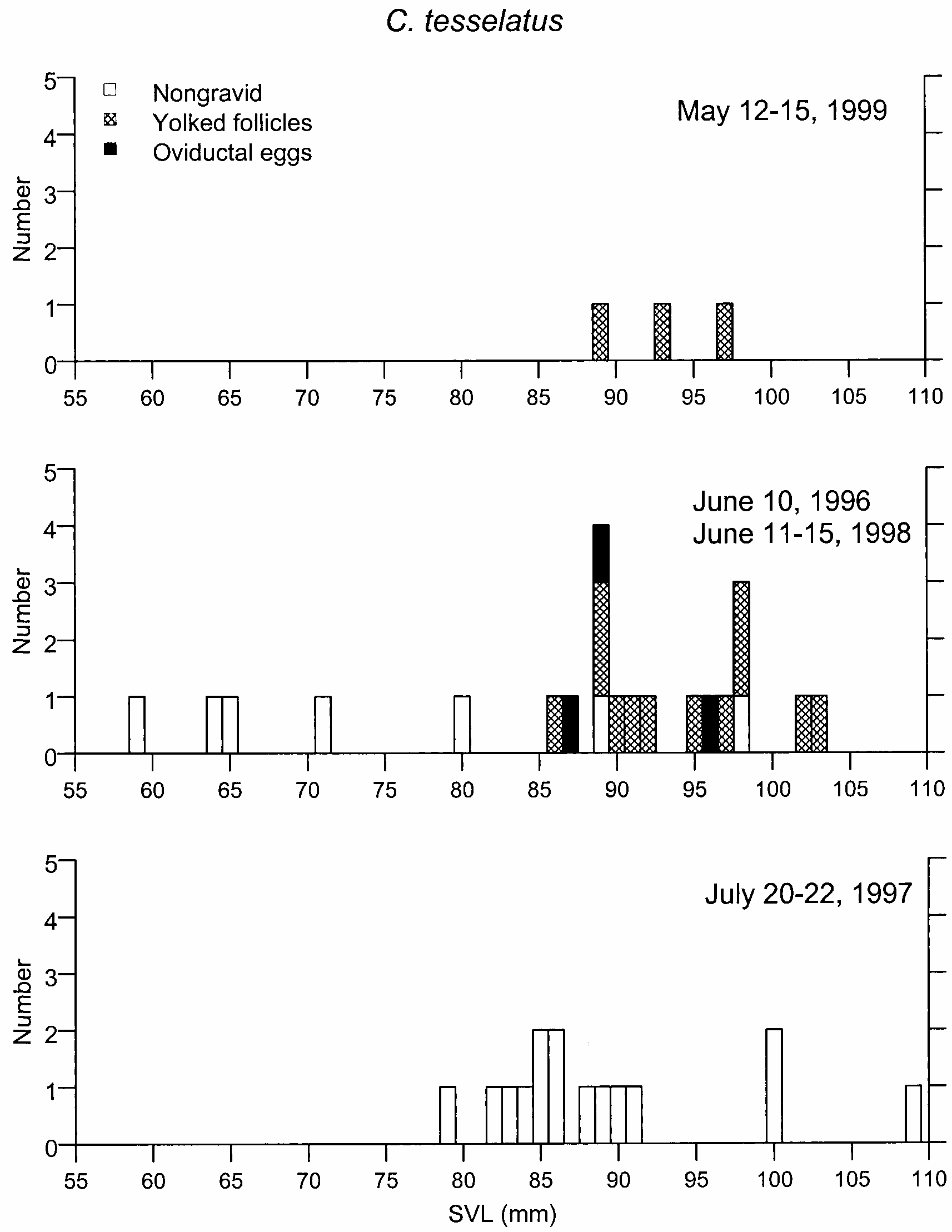
AMNH R146614 ( July 20, 1997): This specimen contained two small corpora lutea and very small follicles (table 6). Like all others collected in July 1997 ( fig. 21 View Fig , table 6), this individual was postreproductive .
AMNH R146618 ( July 20, 1997): This specimen contained a recently ovulated follicle ( fig. 14K View Fig ), several normal follicles with germinal epithelium ( fig. 15F View Fig ), and two oocytes in one follicle (future twins that might share the same eggshell; fig. 15G View Fig ). This follicle would have been available for the 1998 reproductive season. No sperm were found .
AMNH R146620 ( July 21, 1997): A large (7.0 X 3.7 mm) yolked atretic follicle ( fig. 14C View Fig ) and a recent corpus luteum with visible rupture site ( fig. 14J View Fig ) suggest that one of the two large oocytes ovulated and the other aborted, perhaps within two weeks (Miller, 1948) prior to collection. No sperm were found .
AMNH R146621 ( July 21, 1997): An atretic follicle ( fig. 14D View Fig ) and two corpora lutea ( fig. 14L View Fig ) were present. No sperm were found .
follicles containing oocytes (AMNH R146619, slide 3, row 1, section 3, MT). F. Follicle edges of a normal yolked follicle (upper right), atretic yolked follicle (lower right), and normal nonyolked follicle (left; AMNH R146613, slide 6, row 2, section 9, MT). G. Previtellogenic follicle (right) and vitellogenic follicle (left) showing differences in cytoplasm, yolk, and theca development (AMNH R146613, slide 6, row 2, section 6, MT). H. Theca granulosa of normal developing oocyte (AMNH R146629, slide 19, row 1, section 3). Scale bar, 0.1 mm, except C, D, G, H, which are 0.01 mm.
AMNH R146640 ( June 12, 1998): This specimen contained enlarged oviducts but
tiny (unrecognizable macroscopically) ovaries. Many spaces were present around follicle cells and the theca granulosa was almost absent; however, there were no follicle cells in the cytoplasm of the oocytes, which appeared normal ( fig. 18C View Fig ). The adrenal and mesonephric tubules appeared normal (not enlarged) for a female ( fig. 18C View Fig ). No sperm were found.
Other specimens examined did not vary conspicuously from the above descriptions. Important to the question of hybridization frequency, none of the specimens of C. tesselatus had sperm in the oviducts.
During development the ovary is attached to the surface of the adrenal. In its earliest stages (during and soon after the indifferent stage) the ovary appears as a thickened epithelium, almost always with a distinctive lip on one edge. This ovarian lip makes the extremely young ovary more easily recognizable (see Hardy and Cole, 1981: fig. 3 View Fig , top right corner, fig. 4 View Fig , lower right corner, and fig. 6, lower right corner; Hardy and Cole, 1998: fig. 3B View Fig , top center, and fig. 2 View Fig ). In some samples the ovary is so small that only a few oocytes are present. The scarcity of oocytes ( figs. 15F View Fig , 16B, E View Fig ) and small size of the immature ovary make it a difficult organ to recognize. Thus, one must focus on the shape of the ovarian lip, plus the presence of oocytes, in order to recognize the small ovary ( fig. 15A–E View Fig ).
Vitellogenesis in C. tesselatus begins when the follicles are between 3.1 mm (smallest yolked oocyte measured) and 2.3 mm (largest nonyolked oocyte measured) in diameter (using largest dimension of oocyte [table 6]); however, if vitellogenesis is well under way for a normal complement of eggs (2–3 per ovary), then vitellogenesis might be delayed for other oocytes even though they are within the minimum size range for initiation of vitellogenesis.
The two oocytes in one follicle ( fig. 15G View Fig ) could have been independently derived from two different oogonia; a mutation in one oogonium but not in the other would result in these two oocytes being genetically different (one preserving the mutation). Alternatively, they could be the daughter cells, fol lowing endomitotic duplication, of a single oogonium, and mutation in the oogonium
would be contained in both daughter oocytes.
Rupture of the follicle during ovulation would probably result in both oocytes moving as a unit, held together by the theca interna, into the oviduct and thence becoming incorporated within a single eggshell. Development of each oocyte to hatching would produce two hatchlings from a single shell—apparent twinning. However, if the oocytes separated after ovulation but before eggshell formation so that each had its own eggshell, then the potential twinning event would never be detected.
The number of normal yolks produced by a mature female in a season (about five, see below) is probably determined by the energy required for yolk synthesis and the available food supply. How the yolk is packaged is independent of the food supply and reflects the anatomical constraints of the lizard (is the egg too large to be laid?). Containment of both embryos in the same eggshell would probably result in a much larger egg (if both had a normal yolk reserve); this could be detrimental to the mother—the only significant evolutionary consequence of the apparent twinning.
All except 1 of the 26 adult C. tesselatus females had developing follicles and oocytes, and 4 were nearly ready for oviposition (oocyte diameters greater than 6 mm). One female (AMNH R146636; snout–vent length = 95 mm) was nonreproductive, evidently because of developmental failure. This specimen had a snout–vent length large enough that one would expect it to contain enlarged oocytes, but it had no follicles, only a thin ovarian epithelium (almost an indifferent stage of embryonic development; fig. 15E View Fig ), no corpora lutea, no increase in collagen, no evidence of atretic follicles, and a tiny oviduct, whereas the smallest female (AMNH R146640; snout–vent length = 65 mm) had at least 10 enlarged oocytes (maximum size = 0.3 X 0.5 mm) in follicles that appeared normal ( fig. 18C View Fig ). With the exception of AMNH R146636, these specimens of C. tesselatus appeared to be typical females in all details.
| AMNH |
American Museum of Natural History |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.