Haematotropis Jeekel, 2000
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5064.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:65B0A21A-8B8D-4B55-B6F0-8BE60EB8D3BC |
|
DOI |
https://doi.org/10.5281/zenodo.5815164 |
|
persistent identifier |
https://treatment.plazi.org/id/03876671-FFF5-153B-13C5-CD12FBA6A5E4 |
|
treatment provided by |
Plazi |
|
scientific name |
Haematotropis Jeekel, 2000 |
| status |
|
Genus Haematotropis Jeekel, 2000 View in CoL
Haematotropis Jeekel, 2000: 71 View in CoL , 72.
Haematotropis: Golovatch et al. (2004: 57) View in CoL (key).
Type species: Polydesmus callipus Peters, 1864 , by original designation.
Diagnosis. Paranota of rings 2–4 ( Fig. 2G View FIGURE 2 ) or 2–17 ( H. dentata sp. nov., Fig. 24A–D View FIGURE 24 ) with evident anterolateral teeth; base of the telson broad and hemispherical ( Fig. 3D View FIGURE 3 ). Males of Haematotropis differ from other Aphelidesminae by having the acropodite region expanded medially, cup-shaped in ventral view, with a concavity and cavity in the ventral region ( Fig. 4D View FIGURE 4 ); the distal region is curved ventrally.
Redescription.
Size and form. Adults 30–70 mm long and 4–10 mm wide, with 20 body rings (including telson).
Coloration (long-preserved in 70% ethanol). Body light brown or dark brown and middorsal spots on the metazonite; paranota yellow, orange, reddish, light brown or dark brown; antennae, legs and telson brown, orange or yellow.
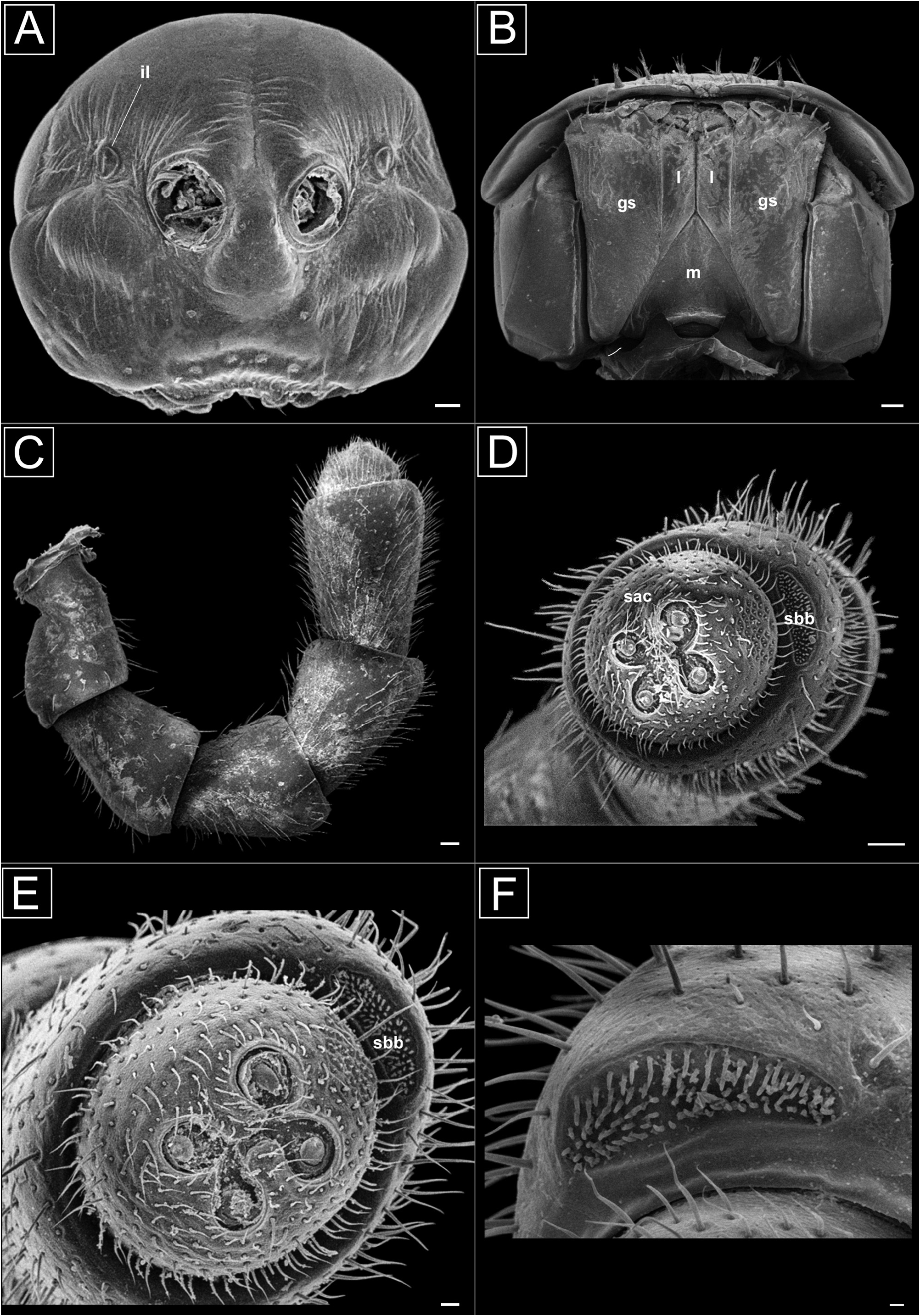
Head. Ventrally inclined; anterolateral and lateral regions strongly to slightly convex, generally smooth and shiny, coriaceous around subovoid incisura lateralis (il) ( Fig. 1A View FIGURE 1 ); labrum slightly emarginate, with three small teeth; base of antenna irregular on lateral surface ( Fig. 1A View FIGURE 1 ). Frons with two setal tufts, strongly or slightly elevated; two and eight setal tufts in clypeal and clypeo-labral areas, respectively ( Fig. 1A View FIGURE 1 ); gnathochilarium rectangular plate formed by fusion of the mentum (m), the paired gnathochilarial stipetes (gs) and the paired lingual lobes (l), covered with setae distally ( Fig. 1B View FIGURE 1 ). Antenna extending posteriorly to around midlength of collum, covered with small setae; setae sparse on antennomeres one to three; 2=3=4=5<6>1>7; 1st antennomere subglobose, 2nd–5th clavate, 6th longest, 7th short and truncate ( Fig. 1C View FIGURE 1 ); terminal antenomere with double invagination between the four sensory apical cones (sac), with a set of sensilla basiconica bacilliformia (sbb) ( Fig. 1D–F View FIGURE 1 ). Interantennal isthmus about 1x diameter of antennal socket.
Trunk. Collum length (mm) 1.3–3.9, width 3.4–9.3. Dorsal surface not glossy ( H. callipa ) or shiny in all rings (other species), with microgranulations ( H. callipa ) or without microgranulations (other species), with medial, horizontal or crescent-shaped depression ( Fig. 2G View FIGURE 2 ); laterally with inconspicuous wrinkles. Rings 2–19: metazonite and prozonite with smooth integument, shiny, lacking setae, except H. callipa , which has microgranulations. Metazonite with anterolateral teeth on paranota of rings 2–4 ( Fig. 2G View FIGURE 2 ) or 2–17 ( H. dentata sp. nov.); peritremata slightly thickened, elevated above dorsal paranotal surfaces ( Fig. 2E View FIGURE 2 ), with microgranulations on anterior and posterior margins ( Fig. 2E, F View FIGURE 2 ); ozopores positioned caudal of peritrematal midlengths on rings 5, 7, 9–10, 12–13 and 15–19, as in most other Polydesmida , opening laterally. Metazonite sterna and pleura with microgranulations ( Fig. 2B View FIGURE 2 ), except in lateral margins and paranota on rings 2–4; microgranulations present ventrally on body rings 2–18 on sternal and pleural regions ( Fig. 2A–D, F View FIGURE 2 ); wrinkles close to paranota ( Fig. 2C View FIGURE 2 ); those of ring 4 with one pair of rounded lobes, posterior margin of 5th and 6th rings recessed to accommodate gonopodal apices when rings compressed; those of rings 8–18 with two pairs of short, rounded projections on ventral surfaces ( Fig. 2D View FIGURE 2 ). Gonopodal aperture elliptical ( Fig. 2C View FIGURE 2 ). Telson smooth and shiny, except H. callipa whose telson presents microgranulations on dorsal surface; pre-anal ring with two pairs of setal tufts at lateral margins. Paraproctal margins elevated, with two pairs of setae arranged vertically proximal to border. Hypoproct with posterocentral and semilunar composite setae position. Epiproct rounded-spade shape ( Fig. 3D View FIGURE 3 ), in some species with concavity on lateral margins, postero-ventral region with two composite setae and compound and equidistant setae on margin.
Legs. Without microgranulations, except H. callipa ; with short sparse setae on all podomeres, except the tarsus with thick setae clustered in the ventral region. Leg-pairs 1 and 2 short, crassate in males, with many setae on all podomeres, gonapophyses short on all coxae; leg 3 with numerous setae as in legs 4–7; legs 4–7 short and subequal in length; pair of projections in sternite between coxae of legs 3–7. Postgonopodal legs subequal in lengths; tarsal claws short, apex slightly curved.
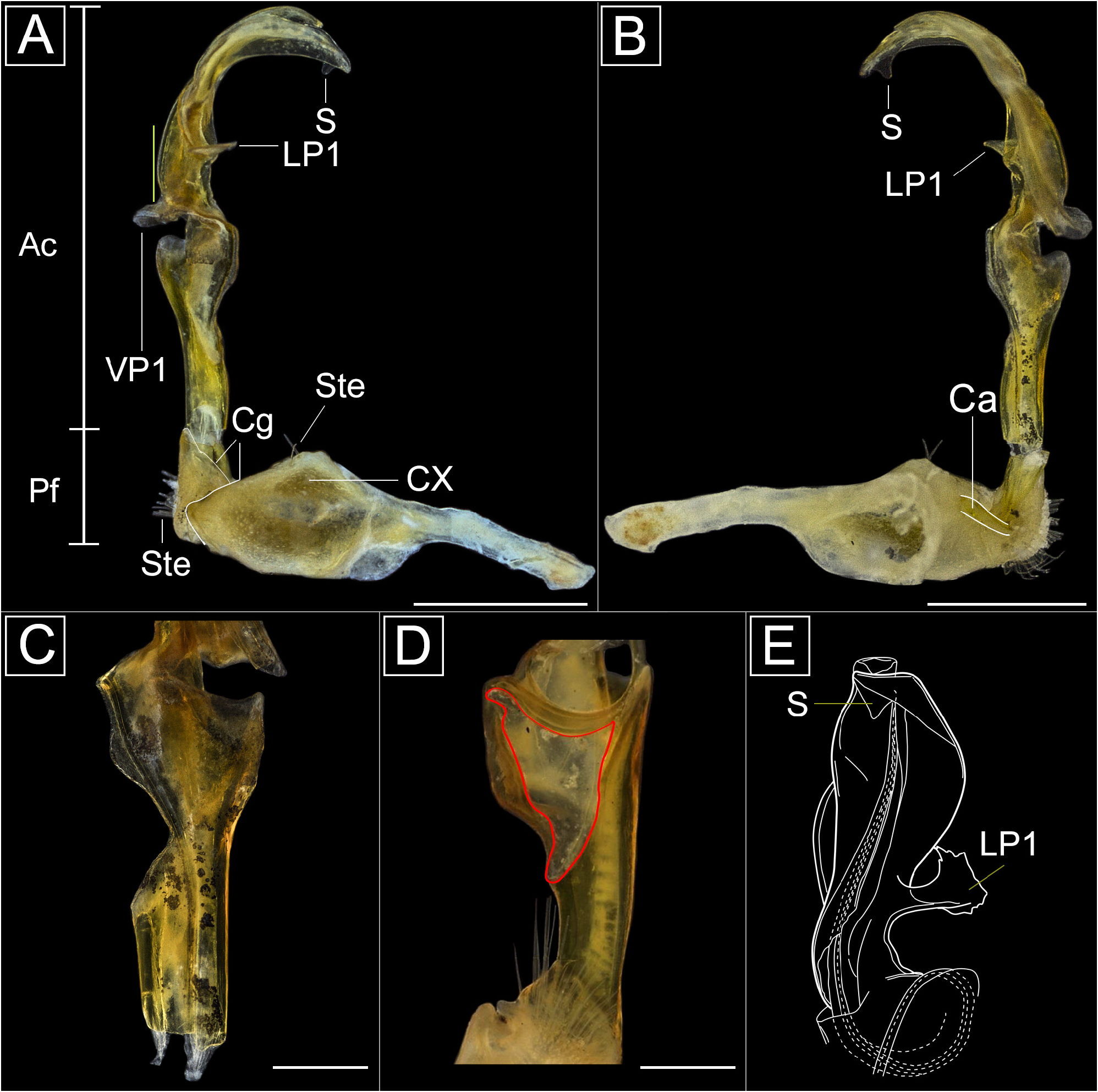
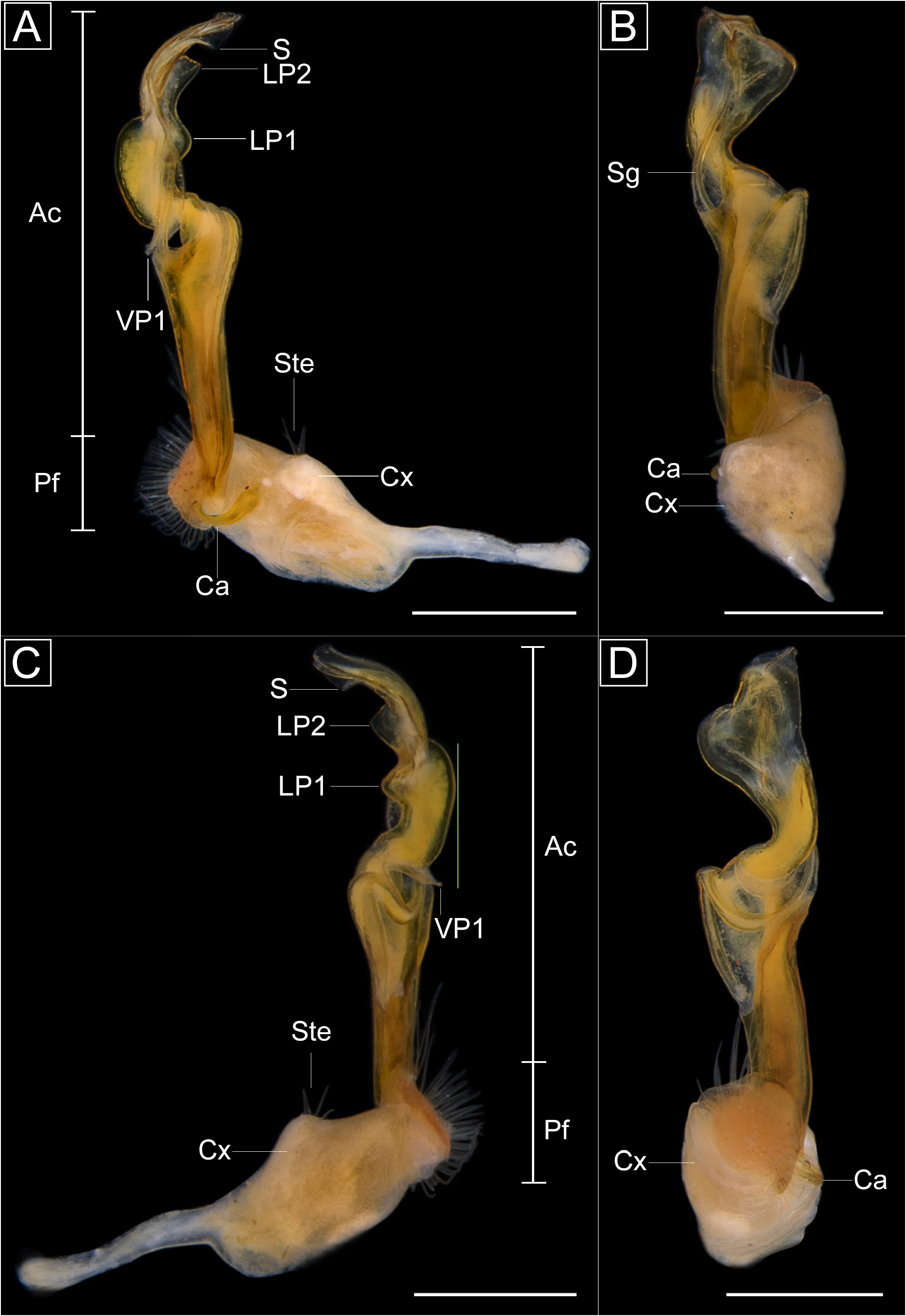
Gonopods. Coxae elongated, usually with lateral swelling at midlength and with setae (Ste) ( Fig. 4A View FIGURE 4 ); cannula (Ca) hook-shaped ( Fig. 4B View FIGURE 4 ). Prefemoral region short, about half the coxa length, delimited by cingulum, densely covered by setae (Ste) around pit-like spermatic groove, setae extending a short distance along acropodite, about ¼ of the length; presence of cingulum (Cg) delimiting the prefemur and acropodital region ( Fig. 4A View FIGURE 4 ). Acropodite region expanded medially, cup-shaped in ventral view, with concavity and cavity ( Fig. 4D View FIGURE 4 ); VP1 present; distal region curved ventrally, LP1 present or absent ( H. dentata sp. nov.) ( Fig. 25A, C View FIGURE 25 ) and ( H. divergens comb. nov.) ( Fig. 45B, D View FIGURE 45 ); LP2 present ( H. goeldii sp. nov.) ( Fig. 29A, C View FIGURE 29 ) or absent; LP3 present ( H. divergens comb. nov.) ( Fig. 45B, D View FIGURE 45 ) or absent; DP present ( H. disjuncta and H. disjunctoides sp. nov.) ( Figs 10A–C View FIGURE 10 , 27A–C View FIGURE 27 ) or absent and VP2 present ( H. melgacensis sp. nov.) ( Fig. 35A, C View FIGURE 35 ) or absent. Opening of the solenomere may or may not be located at distal end of acropodite. Spermatic groove (Sg) extends directly from cannula, through prefemur, continues towards the lateral of acropodite, describing concavity on median region ( Fig. 4D, E View FIGURE 4 ), extends into inner telopodite ( Fig. 6A View FIGURE 6 ), runs through lateral region of acropodite, finally reach solenomere.
Remarks. Based on the material here examined, the gonopods of Haematotropis vary regarding the presence of LP1 and other processes, and characteristics of the telopodite such as the cup-shape in ventral view, and a concavity ( Fig. 4D View FIGURE 4 ). There is also variation in the presence of anterolateral teeth on the paranota of rings 2–4 ( Fig. 2G View FIGURE 2 ), which may be present in other rings. For instance, the paranota of rings 2–17 bear evident anterolateral teeth in H. dentata sp. nov.
The presence of anterolateral teeth in Haematotropis had already been observed by Golovatch et al. (2004), and in Xanthotropis Almeida, Shelley & Rafael, 2018 ( Almeida et al. 2018). These two genera differ mainly because in Haematotropis , the anterolateral teeth are evident; the paranota are more posteriorly projected, the base of the telson is more hemispherical, and the acropodital region of the gonopods is curved ventrally, with the median region expanded, cup-shaped in ventral view, with a concavity ( Fig. 4D View FIGURE 4 ). Xanthotropis has inconspicuous anterolateral teeth; a sublinear posterior margin of the telson and an elongated, distally expanded and laminate gonopodal acropodite, not curved ventrally ( Almeida et al. 2018).
The genus Haematotropis displays great colour variation: light brown to dark brown, with or without middorsal spots on the metazonite, paranota, telson and appendices yellow, orange, reddish, light brown or dark brown. Therefore, the gonopods are still the main structures used for the species differentiation in Polydesmida .
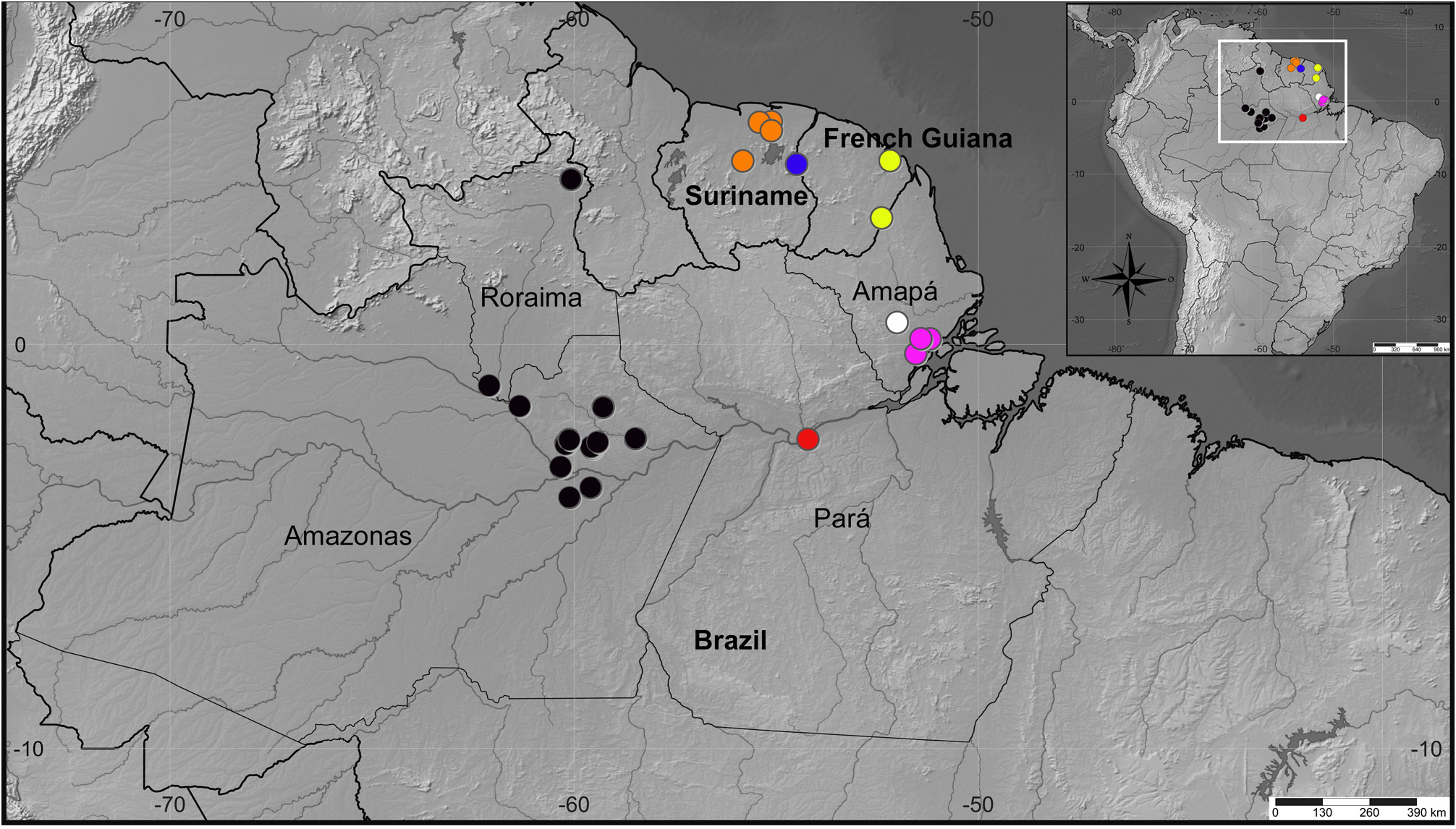
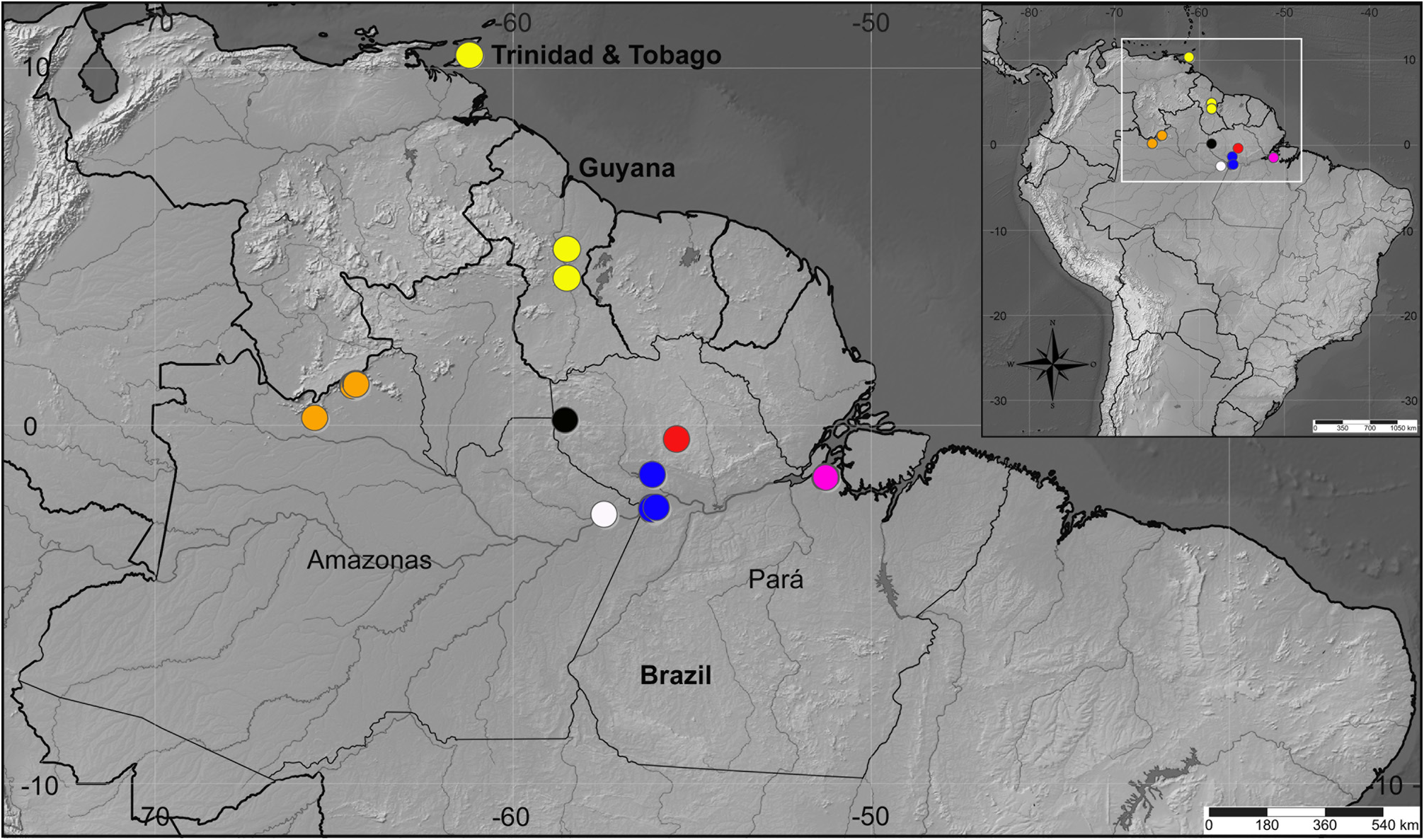
Distribution. Neotropical Region: French Guiana; Suriname; Trinidad & Tobago (new record); Brazil: Amapá, Amazonas, Pará, Mato Grosso (new record), Maranhão (new record) ( Figs 50–52 View FIGURE 50 View FIGURE 51 View FIGURE 52 ).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Haematotropis Jeekel, 2000
| De, Thaís M., Bueno-Villegas, Almeida Julián & Rafael, José A. 2021 |
Haematotropis:
| Golovatch, S. I. & Hoffman, R. L. & Adis, J. & Spelda, J. & Vohland, K. & Seitz, D. 2004: ) |
Haematotropis
| Jeekel, C. A. W. 2000: 71 |