Sophora microphylla, Aiton, Aiton
|
publication ID |
https://doi.org/10.1016/j.phytochem.2015.07.019 |
|
DOI |
https://doi.org/10.5281/zenodo.10530881 |
|
persistent identifier |
https://treatment.plazi.org/id/0388878C-FFA3-DF65-A83F-4C3C9023FA24 |
|
treatment provided by |
Felipe |
|
scientific name |
Sophora microphylla |
| status |
|
2.4. Alkaloid distribution in S. microphylla View in CoL plant parts
Our comparison of the leaf and seed alkaloid compositions across kōwhai (see above) showed that leaf samples contained greater relative amounts of biosynthetic intermediates (A, DHM, and L), versus seed, which generally contained higher amounts of biosynthetic end products: C, NMC, and M ( Table 1 View Table 1 and Fig. 1 View Fig ). These findings are consistent with reports of quinolizidine alkaloid biosynthesis beginning from lysine, occurring in leaf, and metabolic transformation to biosynthetic end-points taking place in seed ( Wink, 1992).
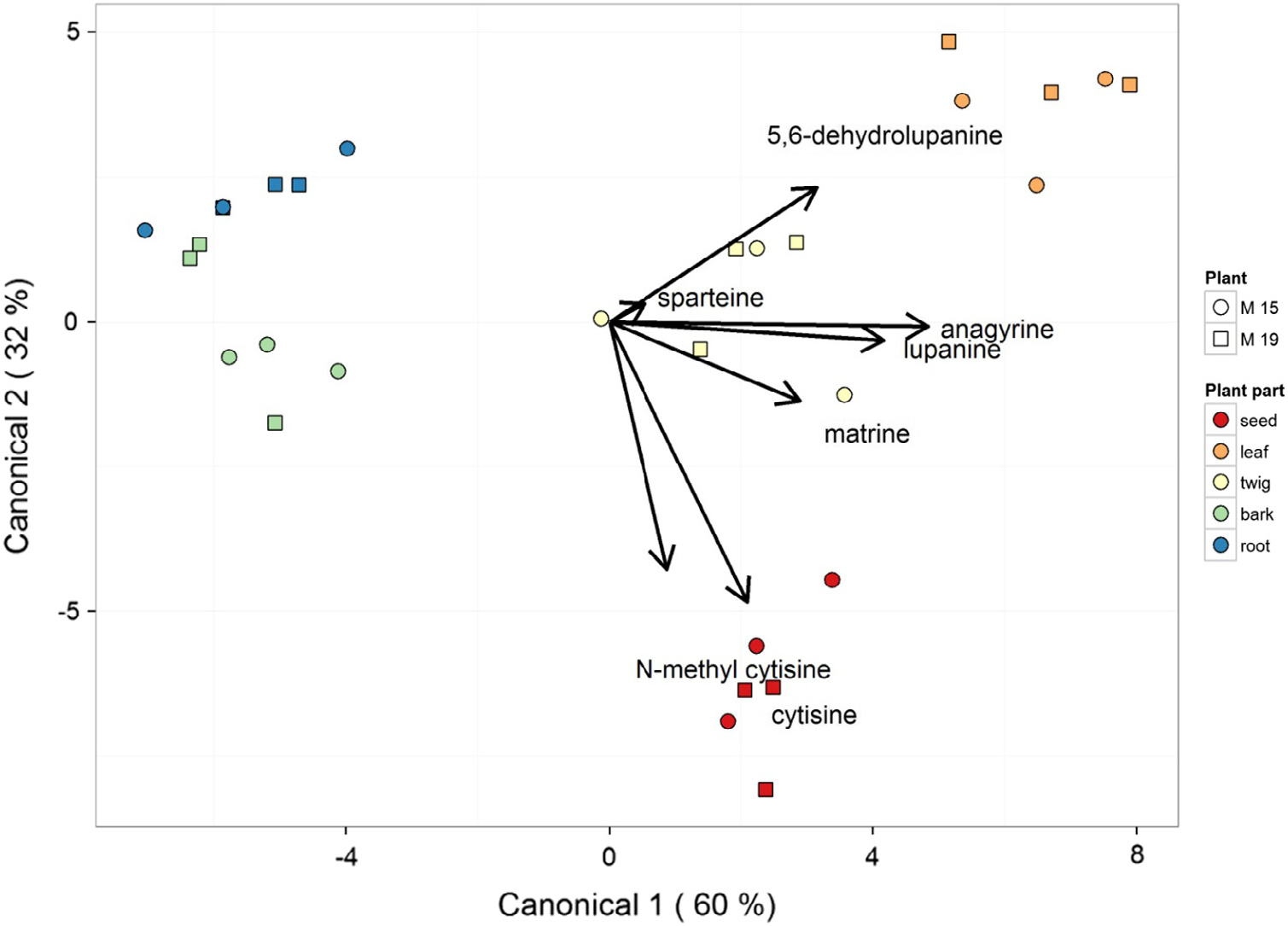
We examined this further by analysing alkaloids from the root, bark, twig, leaf and seed from two individual S. microphylla trees (samples m15 and m19). Integration of the GC-FID alkaloid absolute peak area allowed reasonable approximation of total alkaloid quantities. This was a viable approach because the mass of plant material extracted was within ±0.5% across all samples and the extraction method was consistent for all samples as verified by check, control, and blank samples. The results of the relative alkaloid quantity comparison by plant part are presented in Table 4 View Table 4 for two trees that were found to contain among the highest alkaloid content in the leaves of the 56 leaf extracts studied. In general, seeds contained the highest concentration of alkaloids (supportive of Section 2.3, above). Leaves and twig samples had the next highest concentration of alkaloids followed by bark, with the root samples having the lowest amounts of total alkaloids. The findings presented here reinforce reports in the literature that alkaloids are predominantly concentrated in seed ( Connor, 1977; Burrows and Tyrl, 2001). Compositional analysis of the S. microphylla plant parts showed that the alkaloids differentially accumulated in the various plant parts ( Table 4 View Table 4 ). A MANOVA confirmed the significant ( P <0.01) differences in plant parts alkaloid composition, with a univariate ANOVA test for each alkaloid showing significant differences in the amounts of anagyrine, ammodendrine, cytisine, dehydrolupanine, lupanine and N -methyl cytisine. A canonical discriminate analysis on this data showed clear groups ( Fig. 2 View Fig ) and the contribution of each alkaloid to the dataset variation. The first canonical variable explained 60% of the variation; the second canonical variable explained 32% of the variation. The canonical structure coefficients (as indicated by the arrows on Fig. 2 View Fig ) show that the variations in anagyrine and lupanine have the highest impact on the first canonical variable, followed by dehydrolupanine and matrine. For the second canonical variable N -methylcytisine and cytisine have the highest contributions, followed by dehydrolupanine and then matrine.
The quantitative analysis of total alkaloid content across all plant parts demonstrates that alkaloids are concentrated in the seeds, with minimal amounts in the root and bark, and intermediate quantities in the twig and leaf ( Table 4 View Table 4 ). Plants that produce quinolizidine alkaloids as allelochemicals in their leaves and seeds tend to preferentially produce antifeedant and antifungal phenolics (flavonoids, isoflavonoids, anthocyanins, etc.) in their roots to deter insect herbivores ( Wink, 1992). A complementary phytochemical investigation of phenolics produced by kōwhai is underway. This may validate the prediction that the roots produce allelochemicals equivalent to, or in excess of, those produced in the leaf and seed.
Since many of the nicotinic alkaloids are known to be toxic ( Schep et al., 2009), the amount of alkaloids in Sophora species has potential health concerns in term of intentional or accidental ingestion. Cytisine, for example, has a lethal (LD 50) oral dose of 101 mg /kg in rats ( Barlow and McLeod, 1969), and reports of fatal human poisoning (see ( Musshoff and Madea, 2009) and references therein) corresponding to about 50 mg of cytisine, but the lethal dose in humans is unknown. However, since the seeds have a very hard outer casing, it has been recognised that they would need to be crushed or soaked to poison, otherwise they would pass though the gastrointestinal tract without causing toxicity ( Slaughter et al., 2012).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |