Ovia, Sankaran, Pradeep M., Malamel, Jobi J. & Sebastian, Pothalil A., 2017
|
publication ID |
https://doi.org/10.5281/zenodo.250313 |
|
publication LSID |
lsid:zoobank.org:pub:00F30C0A-66C0-4E33-9306-22E352D867D6 |
|
DOI |
https://doi.org/10.5281/zenodo.4618981 |
|
persistent identifier |
https://treatment.plazi.org/id/038887B2-FFD5-FFC3-C6C5-F001F8C9403B |
|
treatment provided by |
Plazi |
|
scientific name |
Ovia |
| status |
gen. nov. |
Ovia View in CoL View at ENA gen. nov.
Type species: Pardosa procurva Yu & Song, 1988 .
Etymology. The genus name is derived from the Latin word for sheep, ovis , and refers to the characteristic appearance of the anterior pockets of the epigynum, which resemble the horns of the females of certain species of sheep (e.g., Ovis canadensis Shaw, 1804 and Ovis dalli Nelson, 1884 ). It is considered feminine.
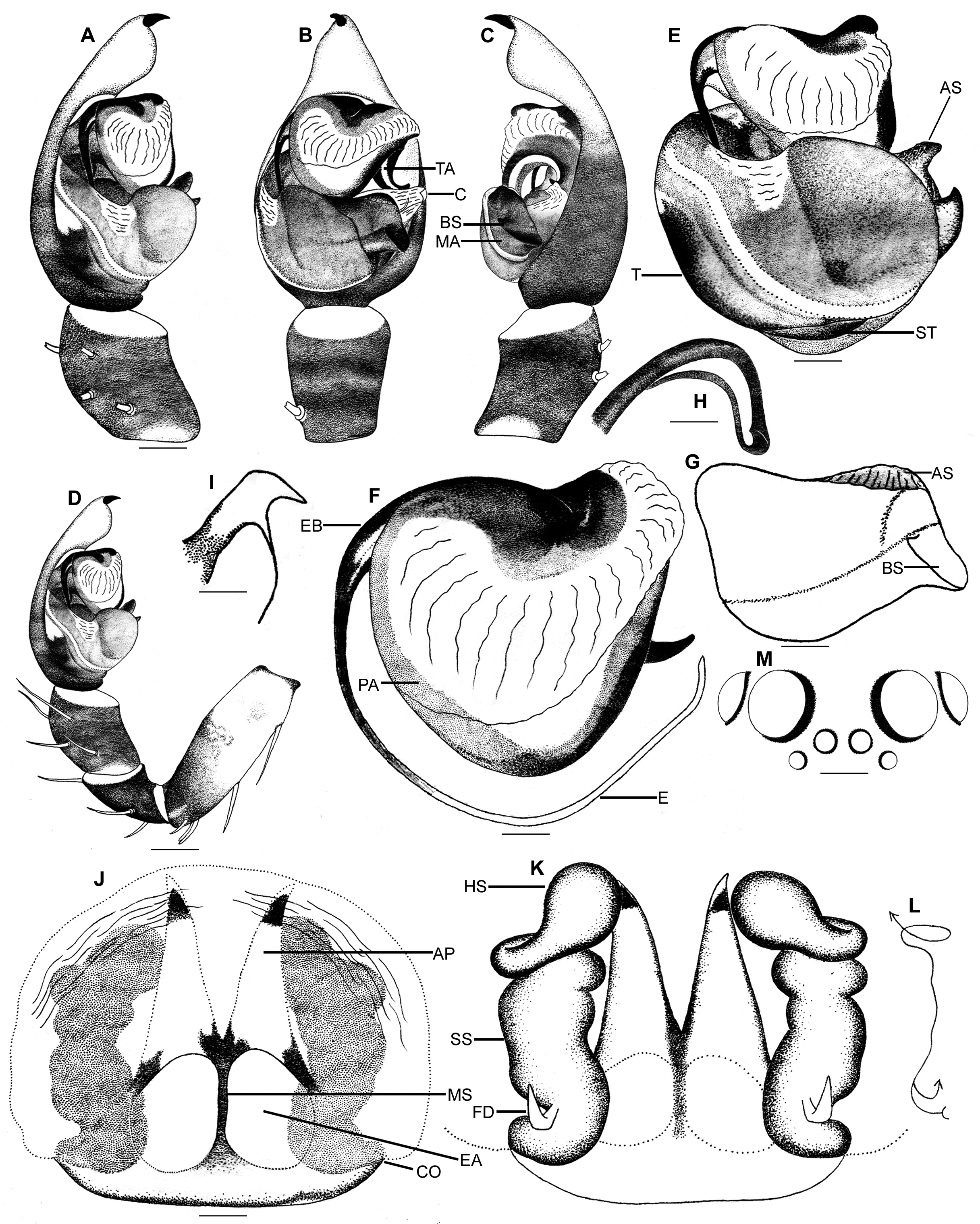
Subfamily placement. Within the family Lycosidae , the subfamily Lycosinae is supported by the male copulatory bulb with a transverse median apophysis with a ventrally directed spur and a sinuous channel dorsally ( Dondale 1986: figs 10, 12–13). The median apophysis of the pedipalp of Ovia gen. nov. shows these features ( Figs 3 View FIGURE 3 D, H, K–M, 5G), indicating that Ovia gen. nov. belongs to Lycosinae.
Diagnosis. Ovia gen. nov. resembles Alopecosa Simon, 1885 within the subfamily Lycosinae (see relationship below). The only putative synapomorphy proposed for Ovia gen. nov., which clearly demarcates this genus from Alopecosa , is the presence of an apical process (spur) on the median apophysis ( Fig. 5 View FIGURE 5 E, G). Both males and females of Ovia gen. nov. differ from those of Alopecosa by having the cheliceral retromargins with three teeth ( Fig. 2 View FIGURE 2 I–J). Males can be further distinguished by the following combination of features: subtegulum small and hardly visible ventrally, palea large and dorso-ventrally flattened, terminal apophysis large and hook-like, conductor with distal constriction, a very long embolus with highly reduced semi-transparent laminar extension on concave side, and a non-angular median apophysis ( Figs 3 View FIGURE 3 D–E, G, I–M, 5B–C, E–G, I). Females can be recognized by the presence of large anterior pockets/epigynal hoods, absence of epigynal median atrium and enlarged lateral epigynal atria ( Figs 4 View FIGURE 4 A–B, 5J). For details on Alopecosa , see Lugetti & Tongiorgi (1969), Dondale & Redner (1979, 1990) and Almquist (2005).
Description. Small lycosid spiders. Prosoma brownish to blackish, with iridescent scales, with (females) or without (males) a broad median band of whitish to creamy-white setae, with indistinct radial pattern. Anterior eye row slightly shorter than the posterior. Anterior eye row procurved ( Fig. 5 View FIGURE 5 M), AME slightly larger than ALE. Chelicerae with three promarginal and three retromarginal teeth ( Fig. 2 View FIGURE 2 I–J). Labium as wide as long. Sternum clothed with long black setae. Leg segments covered with iridescent scales. All tibiae and metatarsi without dorsal spines. Scopulae on metatarsi and tarsi absent. Tarsi provided with spinules. Leg formula 4123 (male) and 4132 (female). Opisthosoma dorsally with (females) or without (males) a broad median band of whitish to creamy-white setae, dorsally and laterally covered with iridescent scales.
Tip of male cymbium with a large, claw-like macroseta ( Figs 3 View FIGURE 3 A–C, 5A–D). Subtegulum hardly visible in ventral view ( Figs 3 View FIGURE 3 E, 5E). Palea broad, tongue-like, dorso-ventrally flattened and lacking a subterminal apophysis ( Figs 3 View FIGURE 3 I–J, 5F). Terminal apophysis large, hook-like, grooved along the entire length and originating apicoretrolaterally on palea ( Figs 3 View FIGURE 3 B, G, M, 5B, H). Median apophysis transversely oriented with apical and basal processes (spurs—Figs 3D–H, K–M, 5B–C, E, G). Embolus long, filiform, C-shaped, with highly reduced semitransparent laminar extension on concave side, originating apico-prolaterally to palea ( Figs 3 View FIGURE 3 E–F, I–J, 5E–F). Conductor prominent, hyaline, with distal constriction ( Figs 3 View FIGURE 3 H, K, M, 5B–C, I).
Epigynum hirsute, with large paired, lateral atria ( Fig. 4 View FIGURE 4 A–B). Median septum incomplete (i.e., not inverted ‘T’ shaped), not set in median atrium ( Figs 4 View FIGURE 4 A–B, 5J). Copulatory openings small, lying near to or at the posterior borderline of epigynum. Anterior pockets/epigynal hoods prominent, deep, long, reaching up to the anterior borderline of the epigynum ( Figs 4 View FIGURE 4 A–C, 5J–K). Spermathecae highly sclerotised, long, extending up to the tip of epigynal hood, stalk of spermathecae with tenuous convolutions ( Figs 4 View FIGURE 4 C, 5K).
Distribution. Indomalayan region: China, India, Taiwan (Fig. 7).
Species included. Only the type species, Ovia procurva comb. nov.
Relationships. Phylogenetic relationships of Ovia gen. nov. to other lycosine genera are unclear, but it is possibly closely related to the Holarctic genus Alopecosa . Both Ovia gen. nov. and Alopecosa share the following putative synapomorphic features that support a sister-taxon relationship: a flat, transverse median apophysis that masks the median part of embolus, a short, conspicuous conductor oriented between the distal halves of terminal and median apophyses, a filiform embolus, and absence of subterminal apophysis. The new genus proposed herein probably does not belong within a monophyletic group of Alopecosa species, which is supported by a short, slender, lobe-like terminal apophysis (see Lugetti & Tongiorgi 1969: fig. 7b–c, Dondale & Redner 1979: figs 1–3, 16). At this stage, one can hypothesize that Ovia gen. nov. might be of Holarctic origin, supported by the wide distribution of its type species, O. procurva (Fig.7) and the Holarctic distribution of Alopecosa . Once originated somewhere in the Holarctic region, Ovia gen. nov. might have invaded India after it collided with Eurasia some 50 M. Y.A., during the Early Eocene Epoch ( Briggs 1989) before the Grande Coupure Extinction Event (the Eocene- Oligocene Extinction Event).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.