Aceria fraxiniflora ( Felt, 1906 )
|
publication ID |
https://doi.org/10.11646/zootaxa.4568.2.5 |
|
publication LSID |
lsid:zoobank.org:pub:066DCC4E-D2E2-4930-97BA-1C61C00D6C1A |
|
DOI |
https://doi.org/10.5281/zenodo.5943104 |
|
persistent identifier |
https://treatment.plazi.org/id/0388D64F-3A51-FF65-FF7E-AFD1DE02F81D |
|
treatment provided by |
Plazi |
|
scientific name |
Aceria fraxiniflora ( Felt, 1906 ) |
| status |
|
Aceria fraxiniflora ( Felt, 1906)
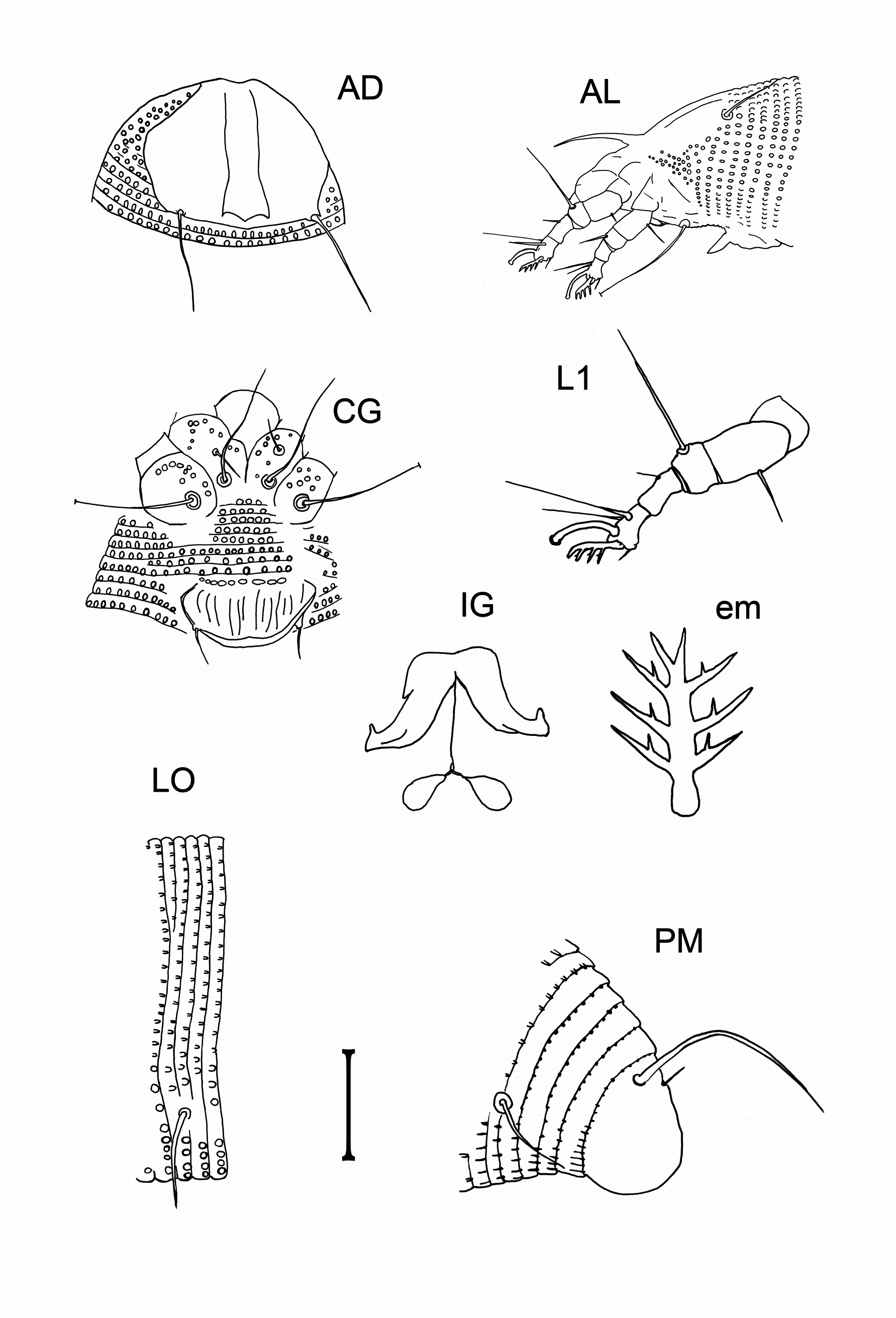
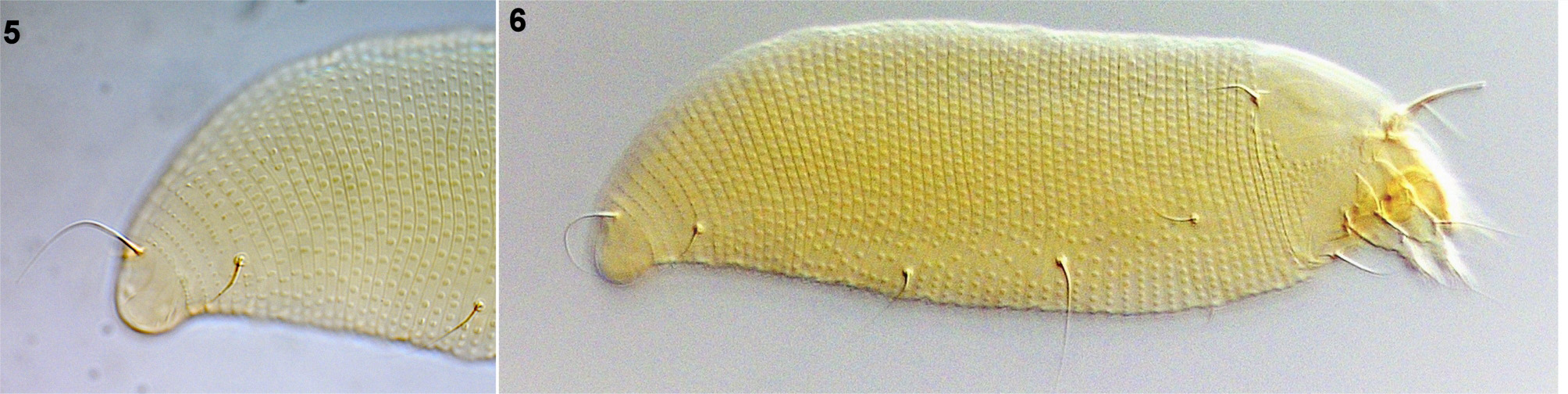
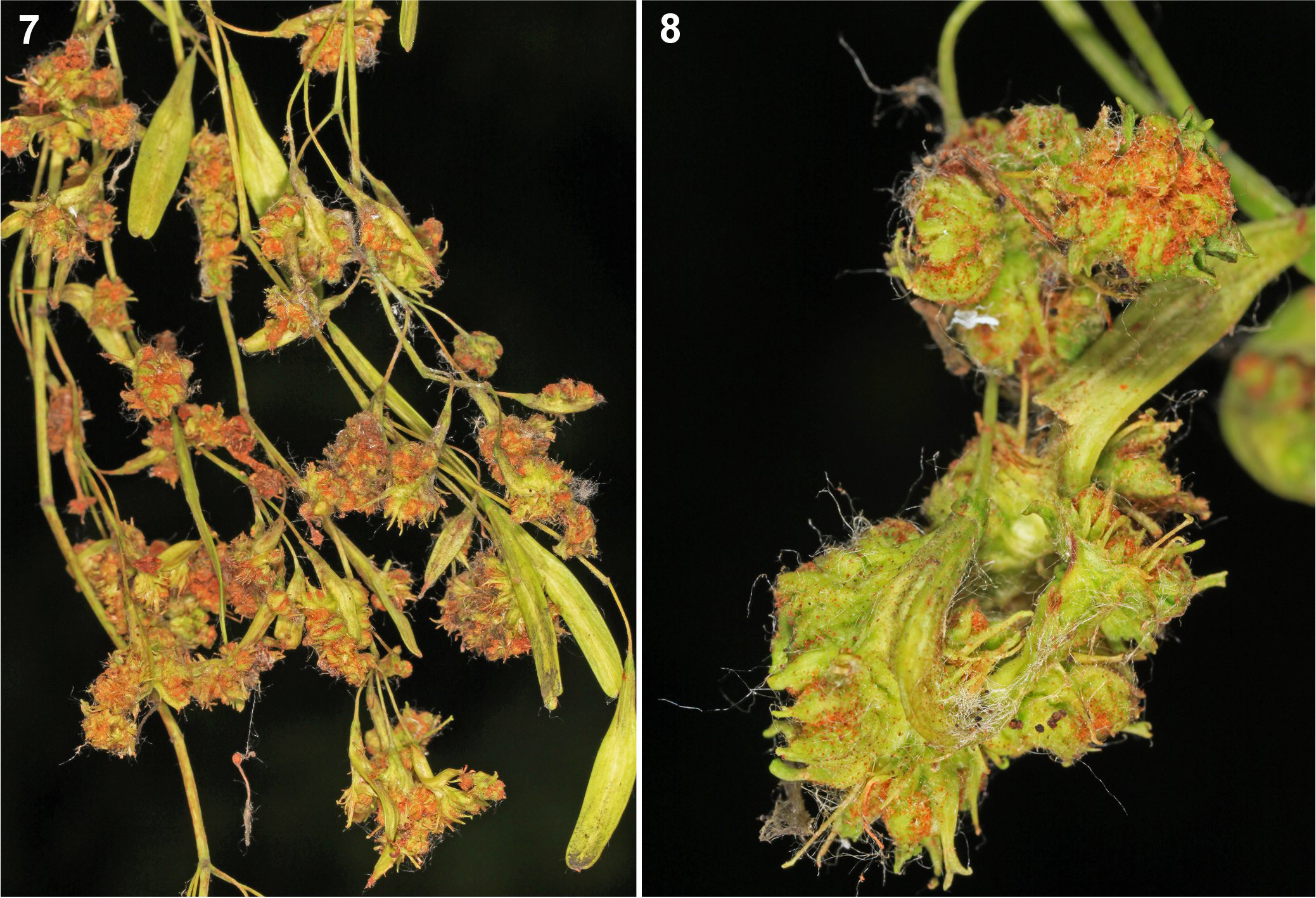
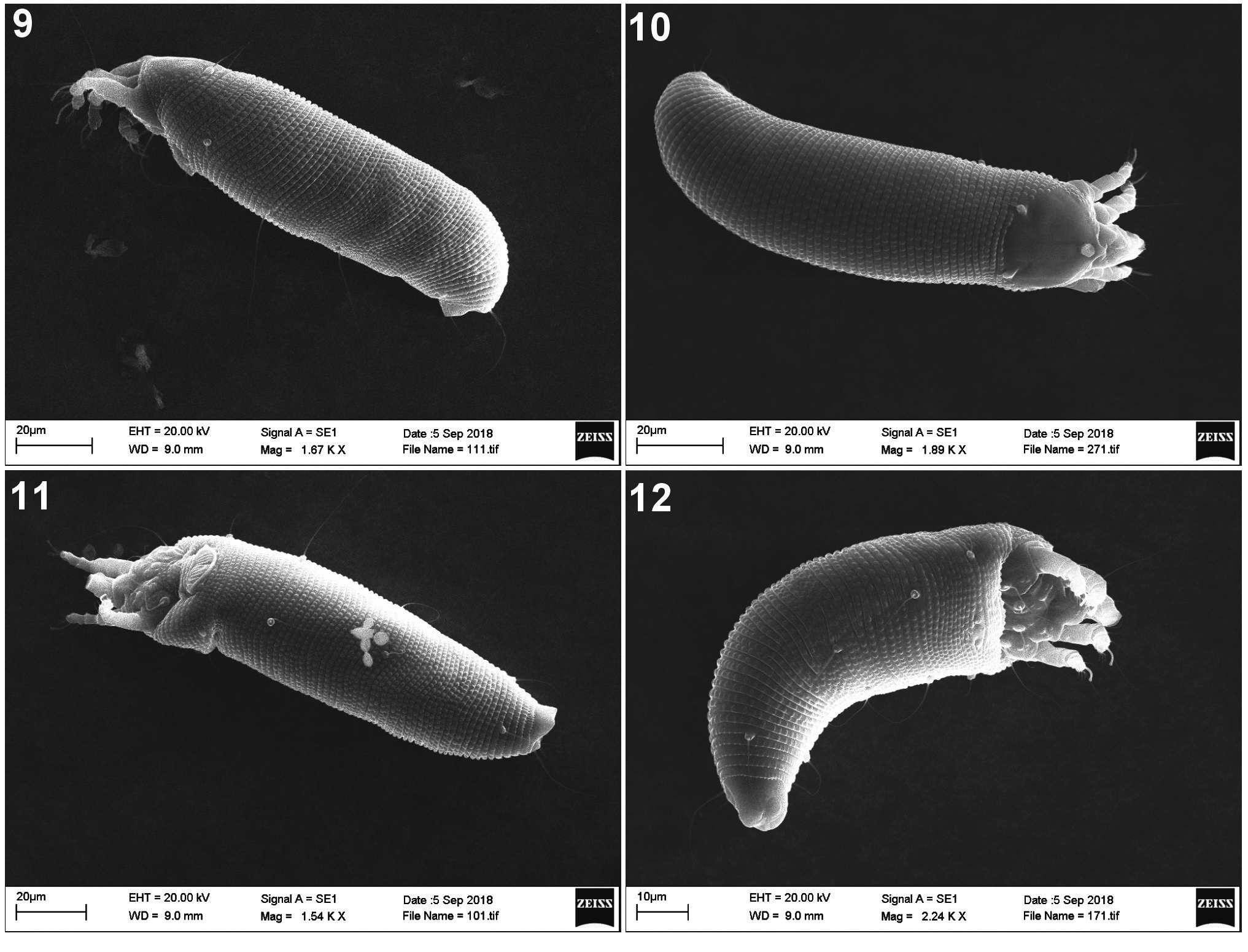
( Figs 1–12 View FIGURE 1 View FIGURES 2–4 View FIGURES 5–6 View FIGURES 7–8 View FIGURES 9–12 )
Eriophyes fraxiniflora Felt, 1906: 633 –634.
Eriophyes fraxinivorus americanus Kendall, 1929: 308 –311, Fig. 4 View FIGURES 2–4 .
Aceria nimia Hall, 1967: 638 –640, pl. 12.
Aceria fraxiniflora (Felt) — Knihinicki & Boczek, 2002: 246 –247, likely misidentification.
From Fraxinus pennsylvanica, Hungarian locality, Békésszentandrás, Hungary ( Figs 1–8 View FIGURE 1 View FIGURES 2–4 View FIGURES 5–6 View FIGURES 7–8 ).
FEMALE. Body pale yellow and ochre, vermiform, 216 (180–248, n = 10), 44 (39–50) wide, 47 (37–54) thick. Gnathosoma 19 (16–22), projecting obliquely downwards; chelicerae 17 (16–20), dorsal palp genual setae d 3 (3–4), unbranched, pedipalp coxal setae ep 3 (3–4). Prodorsal shield 26 (24–27), 31 (26–40) wide, semicircular; shield pattern composed of two relatively faint admedian lines, diverging posteriorly, more widely separate on rear ¼, and two arched transverse lines on or close to the rear shield margin between admedians which resemble a shallow and obtuse V-shaped line; median line absent. Ornamentation of prodorsal shield is considerably variable, e.g. size of admedian lines, and the shield design weakly expressed: a part of the population with smooth prodorsal shield, almost without pattern. Tubercles of scapular setae sc on rear shield margin, 22 (20–24) apart, diverging, scapular setae sc 21 (17–23), directed rearwards. Granules situated in lateral rows on epicoxal areas, i.e. laterally between shield margin and dorsal coxae of legs I and II ( sensu Chetverikov & Craemer 2015).
Legs with all usual segments and setae present. Leg I (foreleg) 28 (25–30), femur 8 (7–8), basiventral femoral seta bv 8 (7–10), genu 5 (3–5), antaxial genual seta l’’ 19 (16–22), tibia 5 (4–6), short paraxial tibial seta l’ located at 2 ⁄5 from dorsal base, 3 (3–4), very thin, tarsus 6 (5–6), unguinal tarsal setae u′ 3 (2–3), solenidion ω 7 (7–8), distally knobbed, slightly curved, empodium simple, bilaterally symmetrical, 5 (5–6), 4-rayed, each ray of three basal pairs with additional secondary branches. Trochanter with a small (0.5) and considerably stout spine ventrally near the femoral segment border.
Leg II (rear leg) 25 (23–27), femur 7 (5–8), basiventral femoral seta bv 7 (6–8), genu 4 (3–4), antaxial genual seta l’’ 5 (5–6) very thin, tibia 5 (3–5), tarsus 5 (4–6), unguinal tarsal setae u′ 2 (2–3), solenidion ω 7 (7–8), distally knobbed, slightly curved, empodium simple, bilaterally symmetrical, 5 (5–6), 4-rayed, each ray of three basal pairs with small subdivisions. Trochanter with a small (0.5) and considerably stout spine ventrally near the femoral segment border.
Coxigenital area with 8–9 microtuberculate semiannuli. Coxisternae I and II with several granules; anterior seta on coxisternum I, 1b 7 (5–8), tubercles setae 1b 11 (10–12) apart, proximal seta on coxisternum I, 1a 25 (16– 29), tubercles setae 1a 7 (7–8) apart, proximal seta on coxisternum II, 2a 43 (39–49), tubercles setae 2a 18 (18–20) apart. Subcapitular plate rounded. Prosternal apodeme 6 (5–6).
Opisthosoma with 77 (69–85) dorsal, 70 (67–74) microtuberculate ventral semiannuli. Microtubercles oval dorsally and rounded ventrally. Last 4-5 dorsal annuli with minute microtubercles on rear annular margin. Last 6–7 ventral annuli with linear microtubercles. Opisthosomal seta c2 23 (20–27), on annulus 10 (9–11), 40 (33–45) apart; opisthosomal seta d 55 (50–59), on annulus 22 (19–24), 29 (26–33) apart; opisthosomal seta e 37 (32–42), on annulus 37 (32–41), 20 (17–23) apart; opisthosomal seta f 20 (17–23), on annulus 65 (57–69), or 5 (4–5) from the rear, 19 (18–20) apart. Opisthosomal seta h2 36 (35–40), very thin at apex, 10 (10–11) apart; opisthosomal seta h1 4 (3–5), 6 (5–7) apart. Anal lobes normal.
Genital plate 15 (13–16), 19 (18–20) wide. Female genital coverflap with 12 (10–13) longitudinal ridges; coxisternal III setae 3a 15 (14–16) apart, 8 (7–9), very thin.
NYMPH. White, wormlike, 142–162 (n = 2), 35–52 thick. Gnathosoma 20–21. Prodorsal shield 24–25. Setae sc 11, pointing rear. Leg I 20, femur 5–7, basiventral femoral seta bv 2, genu 2, antaxial genual setae l″ 15–18, tibia 3, paraxial tibial setae l′ located at ¼– 2 ⁄5 from dorsal base, 2–3, very fine, tarsus 3–4, solenidion ω 5–6, slightly curved, distally knobbed, empodium simple, bilaterally symmetrical, 4, with 4 paired rays. Leg II 17, femur 4–5, basiventral femoral setae bv 3, very fine, genu 2–3, antaxial genual setae l″ 2, very fine, tibia 2, tarsus 3, solenidion ω 5, slightly curved, distally knobbed, empodium simple, bilaterally symmetrical, 4, with 4 paired rays. Setae 1b 5–6, setae 1a 9–12, setae 2a 25. Opisthosoma with 70–71 dorsal, 58–67 ventral semiannuli. Dorsal and ventral semiannuli with minute microtubercles. Setae c 2 8–10, on annulus 10–11; setae d 25–33, on annulus 22–23; setae e 10–17, on annulus 33–37; setae f 13–17, on annulus 53–63, or 4–5 from rear. Setae h 2 25–27; setae h1 2; setae 3a 2–3.
Host plant. Green ash, Fraxinus pennsylvanica Marshall (fam. Oleaceae ). Green ash is the most widespread of the North American ash species, native to eastern and central North America, and an invasive transformer tree species in Hungary living in different plant associations, especially along rivers ( Csiszár & Bartha, 2004). Green ash came to Europe in 1780, and around 1802 to Hungary ( Csiszár & Bartha, 2004, Korda, 2018).
Relationship to the host. This mite caused distinct inflorescence and fruit deformation in the shape of a mass of globular sponge-like galls. The panicles swelled and distorted. The mite was found in high number on the pubescent and lumpy samaras and remnants of panicles of the host ( Figs 7–8 View FIGURES 7–8 ).
Hungarian locality. Békésszentandrás, Békés county (Southern Hungary), in a soft-wood gallery forest along river Hármas-Körös, 81 m elev., 46°54'0635'' N, 20°27'4234'' E.
Material examined. The described and illustrated female circled with black ink among 21 females on one slide, 23 July 2017, slide # 1423a, coll. Mr. Márton Korda. Other specimens were collected by Mr. M. Korda in the same locality and time. The 2 slides (## 1423b, 1423c with described nymphs) were prepared from this material containing 30 females and 2 nymphs, and 25 females and 1 nymph, resp. from the fruits of the same host. Slides are in the corresponding author’s collection and deposited in the National Food Chain Safety Office, Directorate of Plant Protection, Soil Conservation and Agri-environment, Department of Pest Management Development and Coordination, Budapest, Hungary .
From Fraxinus americana , North American locality, Terre Haute, Indiana, USA. ( Figs 9–12 View FIGURES 9–12 )
FEMALE. Body yellowish white, vermiform, 216 (174–230, n = 9), 54 (50–60) wide, 52 (50–55) thick. Gnathosoma 19 (18–22), projecting obliquely downwards; chelicerae 17 (16–17), dorsal palp genual setae d 3 (3– 4), unbranched, pedipalp coxal setae ep 3 (3–4). Prodorsal shield 28 (25–28), 30 (25–30) wide, semicircular; with a small frontal lobe 2 (2–3); shield pattern composed of two relatively faint admedian lines, diverging posteriorly, more widely separate on rear ¼; median line absent. Ornamentation of prodorsal shield is considerably variable, e.g. size of admedian lines, and the shield design weakly expressed: a part of the population with smooth prodorsal shield, almost without pattern. Tubercles of scapular setae sc on rear shield margin, 21 (17–23) apart, diverging, scapular setae sc 21 (17–25), directed rearwards. Granules situated in lateral rows on epicoxal areas, i.e. laterally between shield margin and dorsal coxae of legs I and II.
Legs with all usual segments and setae present. Leg I 26 (23–28), femur 7 (7–8), basiventral femoral seta bv 8 (7–10), genu 4 (3–4), antaxial genual seta l’’ 18 (16–21), tibia 4 (3–5), short paraxial tibial seta l’ located at 2 ⁄5 from dorsal base, 3 (2–3), very thin, tarsus 6 (5–6), unguinal tarsal setae u′ 3 (2–3), solenidion ω 7 (7–8), distally knobbed, slightly curved, empodium simple, bilaterally symmetrical, 5 (5–6), 4-rayed.
Leg II 23 (20–25), femur 6 (5–8), basiventral femoral seta bv 7 (6–8), genu 3 (3–4), antaxial genual seta l’’ 5 (5–6) very thin, tibia 4 (3–4), tarsus 5 (4–5), unguinal tarsal setae u′ 2 (2–3), solenidion ω 7 (7–8), distally knobbed, slightly curved, empodium simple, bilaterally symmetrical, 5 (5–6), 4-rayed.
Coxigenital area with 7–8 microtuberculate semiannuli. Coxisternae I and II with several granules; anterior seta on coxisternum I, 1b 8 (7–10), tubercles setae 1b 11 (11–12) apart, proximal seta on coxisternum I, 1a 25 (15– 28), tubercles setae 1a 8 (7–8) apart, proximal seta on coxisternum II, 2a 40 (36–41), tubercles setae 2a 20 (19–20) apart. Subcapitular plate rounded. Prosternal apodeme 6 (5–6).
Opisthosoma with 74 (65–79) dorsal, 70 (64–74) microtuberculate ventral semiannuli. Rounded microtubercles dorsally and ventrally. Last 4–5 dorsal annuli with minute microtubercles on rear annular margin. Last 6–7 ventral annuli with linear microtubercles. Opisthosomal seta c2 18 (13–18), on annulus 10 (10–11), 41 (40–50) apart; opisthosomal seta d 52 (45–54), on annulus 22 (21–23), 29 (25–34) apart; opisthosomal seta e 39 (25–40), on annulus 39 (36–41), 20 (13–21) apart; opisthosomal seta f 20 (17–20), on annulus 65 (59–68), or 5 (4– 5) from the rear, 19 (15–21) apart. Opisthosomal seta h2 30 (25–33), very thin at apex, 11 (10–11) apart; opisthosomal seta h1 5 (4–6), 6 (5–6) apart. Anal lobes normal.
Genital plate 15 (13–16), 19 (18–22) wide. Female genital coverflap with 12 (10–13) longitudinal ridges; coxisternal III setae 3a 15 (14–16) apart, 7 (5–9), very thin.
NYMPH. White, wormlike, 123–167 (n = 2), 42 wide, 38 thick. Gnathosoma 17–20; chelicerae 13. Prodorsal shield 22–25, 25–27 wide, semicircular. Setae sc 15–17, 17 apart, pointing rear. Leg I 18–20, leg II 16–17. Setae 1b 4, 7 apart, setae 1a 10–11, 5 apart, setae 2a 19–22, 13 apart. Opisthosoma with 57–60 dorsal, 57–59 ventral semiannuli. Dorsal annuli with minute and slightly elongate, and ventral semiannuli with tiny microtubercles. Setae c 2 8–10, on annulus 9–10; setae d 14–18, on annulus 20–21; setae e 8–10, on annulus 32–34; setae f 11–13, on annulus 52–55, or 4–5 from rear. Setae h 2 20–23; setae h1 3–4; setae 3a 2–3.
Host plant. White ash or Biltmore ash, Fraxinus americana L. (fam. Oleaceae ) is native to eastern North America, and is a rapidly growing tree suited to parkland plantings, where cultivars selected for yellow, orange or bronze autumn colour are highly valued ( Anonym 2018). White ash is an introduced but not invasive tree species in Hungary ( Korda, 2018). White ash came to Europe in 1724, and in 1798 to Hungary ( Csiszár & Bartha, 2004).
Relationship to the host. This mite caused distinct inflorescence and fruit deformation in the shape of globular sponge-like and rough-surface galls of 1 cm in diameter. The panicles swelled and distorted. The mite was found in high numbers on the lumpy samaras and remnants of panicles of the host.
Locality. Terre Haute, Indiana, USA, 39.461539 N, - 87.418746 E.
Material examined. The examined female circled with black ink among 4 females, 2 nymphs and one male on one slide, 9 July 2018, slide #1446a, coll. Dr. James W. Amrine. Other specimens were collected in the same locality and time, slide # 1446b, was prepared from this material containing 11 females and one nymph from the fruits of the same host. Slides are in the corresponding author’s collection and deposited in the National Food Chain Safety Office, Directorate of Plant Protection, Soil Conservation and Agri-environment, Department of Pest Management Development and Coordination, Budapest, Hungary .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
SuperOrder |
Acariformes |
|
Order |
|
|
SuperFamily |
Eriophyoidea |
|
Family |
|
|
SubFamily |
Eriophyinae |
|
Tribe |
Aceriini |
|
Genus |
Aceria fraxiniflora ( Felt, 1906 )
| Korda, M., Csóka, Gy., Szabó, Á. & Ripka, G. 2019 |
Aceria fraxiniflora
| Knihinicki, D. K. & Boczek, J. 2002: 246 |
Aceria nimia
| Hall, C. C., Jr. 1967: 638 |
Eriophyes fraxinivorus americanus
| Kendall, J. 1929: 308 |
Eriophyes fraxiniflora
| Felt, E. P. 1906: 633 |