Argyresthia Hübner
|
publication ID |
https://doi.org/10.5281/zenodo.827746 |
|
publication LSID |
lsid:zoobank.org:pub:48A417CD-CA76-4CA1-8E2C-93DE2E681CCC |
|
DOI |
https://doi.org/10.5281/zenodo.6051522 |
|
persistent identifier |
https://treatment.plazi.org/id/0389878F-9901-FF98-FF6C-35A5FA820EF6 |
|
treatment provided by |
Plazi |
|
scientific name |
Argyresthia Hübner |
| status |
|
Genus Argyresthia Hübner , [1825]
Argyresthia Hübner , [1825]: 422. Type species: Phalaena ( Tinea) goedartella Linnaeus, 1758 , by subsequent designation.
Argyrosetia Stephens, 1829: 205 . Type species: Phalaena ( Tinea) goedartella Linnaeus, 1758 .
Oligos Treitschke, 1830: 299 . [non descr.] Type species: Phalaena ( Tinea) pruniella Clerke, 1759 .
Ederesa Curtis, 1833: 191 . Type species: Phalaena ( Tinea) pruniella Linnaeus, 1761 .
Ismene Stephens, 1834: 247 View in CoL . Type species: Phalaena ( Tinea) pruniella, Clerke, 1759 .
Blastotere Ratzeburg, 1840: 240 . Type species: Tinea ( Blastotere) bergiella Ratzeburg, 1840 .
Eurynome Chambers, 1875: 304 View in CoL . A junior homonym of Eurynome Leach View in CoL , [1814] [Crustacea]. Type species: Eurynome luteella Chambers, 1875 , by monotypy. [synonymized by Sohn et al., 2015]
Busckia Dyar, 1903: 563 . An objective replacement name of Eurynome Chambers, 1875 View in CoL . [synonymized by Sohn et al., 2015]
Paraargyresthia Moriuti, 1969: 30 . Type species: Paraargyresthia japonica Moriuti, 1969 . [synonymized by Kyrki, 1990]
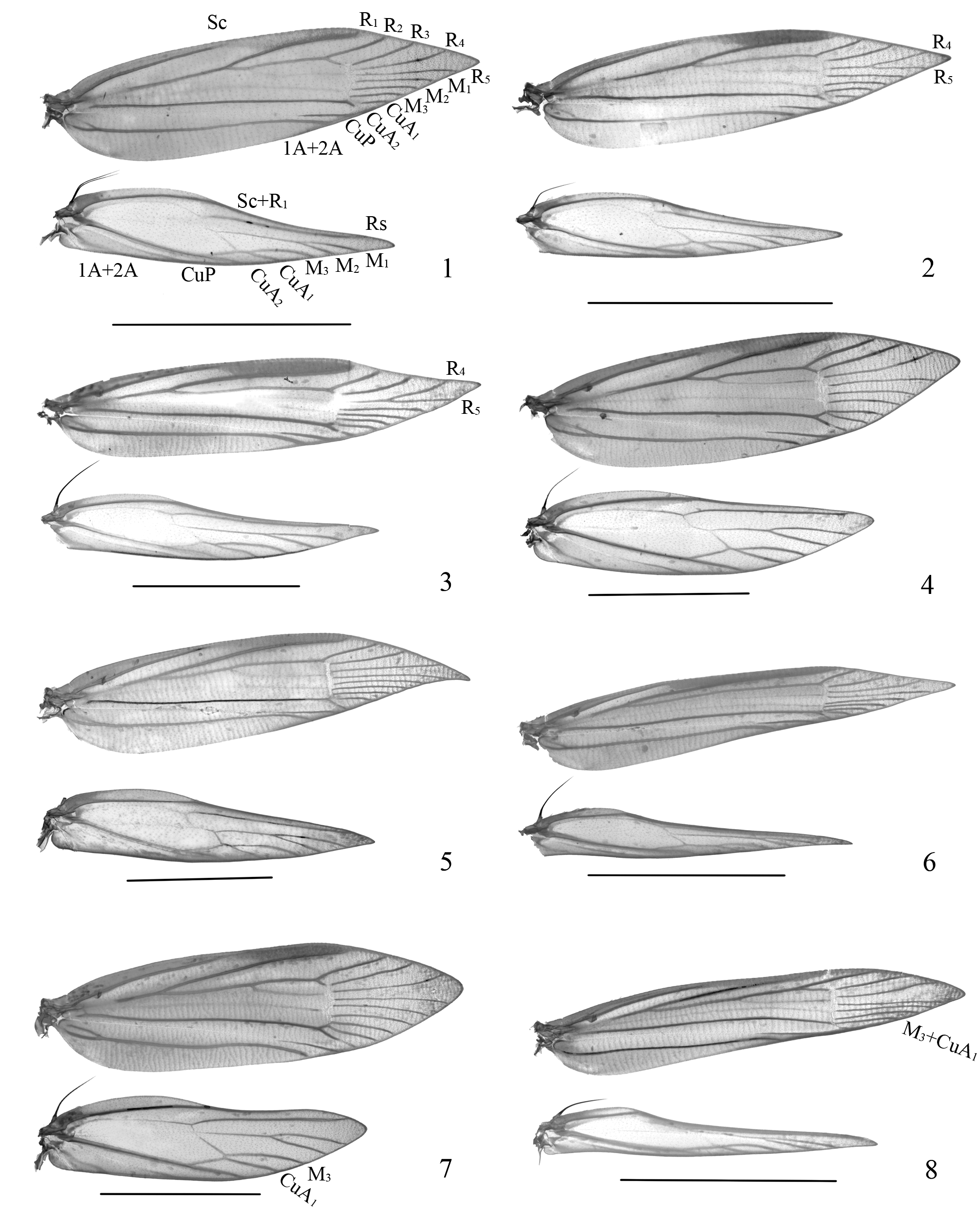
Generic characters. Small to medium-sized glossy moths, with wingspan 7.0–17.0 mm. Head with vertex rough, occiput with upright or appressed piliform scales. Labial palpus pointed or obtuse apically; inner surface usually paler than outer surface. Antenna 3/4̄4/5 length of forewing, scape apparently wider, with pecten; a dark ring or dot on each flagellomere dorsally. Foreleg darker on inner surface, tarsomeres usually with a dark apical ring or dot. Forewing ratio 3.6̄5.5, apex pointed, narrowly rounded, or slightly hooked ( Figs. 1 View FIGURES 1 – 8 ̄8); dorsum usually with an outward oblique dark fascia or a streak from before or at middle of wing, often narrowed towards costa, sometimes absent or reduced to a spot. Hindwing lanceolate, pale gray to dark gray.
Venation ( Figs. 1 View FIGURES 1 – 8 ̄8): Forewing with Sc to costal 1/3 or 2/5; R1 originated from middle or beyond middle of upper margin of cell ( Figs. 1 View FIGURES 1 – 8 ̄2, representing most species in the subgenus Blastotere ), or from before basal 2/5 of upper margin of cell ( Figs. 3, 5 View FIGURES 1 – 8 ̄8, representing most species in the subgenus Argyresthia ), reaching distal 3/10 of costa, R2 from before upper angle of cell to about distal 1/4 of costa, R4 and R5 stalked ( Figs. 2, 3 View FIGURES 1 – 8 ) or separate ( Figs. 1, 4 View FIGURES 1 – 8 ̄8), R5 to termen; M1̄M3 nearly parallel, M3 usually from same point as CuA1, or rarely coincident and forming M3+CuA1 ( Fig. 8 View FIGURES 1 – 8 ); CuA1 from or from above lower angle of cell, CuA2 from or from before lower angle of cell, sometimes reduced; CuP visible distally; 1A+2A with short basal fork, reaching basal 3/5 of dorsum. Hindwing with Sc to middle or before middle of costa; Rs to costa before apex; M1 and M2 stalked, M3 arched, usually stalked or occasionally originating from same point as CuA1 ( Fig. 8 View FIGURES 1 – 8 ), or rarely as a separate vein slightly remote from CuA1 ( Fig. 7 View FIGURES 1 – 8 ), CuA2 from lower angle of cell, CuP usually visible distally; 1A+2A with short basal fork, 3A reduced.
Male genitalia ( Fig. 9 View FIGURES 9 – 10 ): Socius with scale-like setae of relatively fixed quantity, shape, and position within the same species, but diverse among species. Gnathos linear, sclerotized, sometimes inflated distally and with several long, thick setae apically. Valva oval, heart-shaped, or subtriangular, rarely rectangular or knife-shaped, with sparse setae along margin (subgenus Blastotere ), or with a clump or row of long, thick setae in disc (most species of subgenus Argyresthia ), or with dense short setae ( A. ( A.) metallicolor Moriuti, 1969 and A. ( A.) ellipsoidea , sp. nov.). Vinculum simple, often weakly sclerotized, rarely heavily sclerotized ( A. ( B.) japonica (Moriuti, 1969)) ; saccus a pair of varied rods joined basally. Phallus slender; cornuti represented by a long spine and numerous micro spines around the long spine (subgenus Argyresthia ), sometimes with several strong denticles, rarely with many heavily sclerotized spines on distal part of long spine (subgenus Blastotere ).
Female genitalia ( Fig. 10 View FIGURES 9 – 10 ): Anterior apophysis bifurcate (except in A. ( A.) metallicolor ); branch usually extending to and fused with lamella postvaginalis, sometimes shortly bifurcate a second time with its dorsal branch extending to the lamella postvaginalis, ventral branches usually fused and forming ventral margin of ostium bursae. Lamella postvaginalis sclerotized, varied in shape. Antrum spinulose, funnel-shaped, or tubular, connected with ductus bursae by an incomplete sclerotized ring. Ductus bursae long and slender, slightly dilated anteriorly, or short and broad (e.g., A. ( B.) japonica (Moriuti, 1969)) , usually covered with micro-spines near opening of ductus seminalis. Ductus seminalis spinulate, originated from posterior 1/6 to anterior 1/3 of ductus bursae. Corpus bursae elliptical or ovate, sometimes contracted laterally at middle and deeply concave anteriorly, densely denticulate or spinulate, especially on posterior part; signum usually a basal plate with two horns, all denticulate, occasionally with a single horn, or without a horn.
Abdominal structure: Second sternite with two rows of micro-setae, varied in number interspecifically; third to seventh sternites with fewer scattered similar setae. Male sternite VIII Y-shaped, V-shaped, or C-shaped. Coremata usually present and moderately short, but occasionally extremely long or absent.
Diagnosis. Argyresthia resembles Lycophantis Meyrick, 1914 (Yponomeutidae) superficially. It can be separated by the combination of the following characters: the forewing of Argyresthia has more diverse and colorful patterns, whereas that of Lycophantis usually has a golden or dark brown area between costa and the fold, and is white on the remaining area ( Cong & Li 2016). The most useful diagnostic characters are their genitalia. In Argyresthia the socius is covered with scale-like setae and the saccus is paired in the male genitalia, and the signum is usually composed of a basal plate with horn(s) in the female genitalia. In contrast, in Lycophantis the socius is covered with piliform setae, the saccus is singular, and the signum is composed of many granules but lacking horns.
Biology. Adults of Argyresthiidae usually exhibit a characterisitc resting posture ( Fig. 78 View FIGURES 76 – 83 ) with the head close to the surface, the hindlegs alongside the abdomen, which rises from the surface by about 45°, and the antennae parallel to the surface and almost vertical to the body. At least 13 plant families are recorded as host plants of Argyresthia , but hosts are recorded for only about 1/3 of the known species ( Krauss 1965; Silva et al. 1995; Gershenson & Vasiljeva 1996; APG 2009). Twenty-four species feed on Cupressaceae , 21 on Pinaceae , 14 on Rosaceae , six on Fagaceae , five on Betulaceae , two on Ericaceae , two on Salicaceae , and one species each on Ulmaceae , Taxodiaceae , Saxifragaceae , Sapindaceae , Myricaceae , and Melastomataceae .
Despite the fact that most species are recorded from a single plant family, seven biologically well-known species (i.e., A. ( A.) glaucinella Zeller, 1839 , A. ( A.) pulchella Zeller, 1846 , A. ( A.) albistria (Haworth, 1828) , A. ( A.) retinella Zeller, 1839 , A. ( A.) ivella (Haworth, 1828) , A. ( A.) pruniella (Clerck, 1759) , and A. ( A.) goedartella (Linnaeus, 1758)) feed on at least two plant families. Larvae of most species mine shoots and leaves or feed within fruits ( Freeman 1972; Moriuti 1977; Gibeaux 1983b; Agassiz 1996); larvae of A. ( A.) retinella and A. furcatella Busck, 1916 are gall makers ( Busck 1916; Robbins 1992). Several species of Argyresthia (e.g., the apple fruit moth A. ( A.) conjugella Zeller, 1839 ), are economically important. Larvae of A. ( A.) conjugella bore into apple fruit and require about six weeks for larval development ( Agassiz 1996). Argyreshia ( A.) mala , sp. nov., another pest of apple fruits, had been confused with A. ( A.) assimilis Moriuti, 1977 in China since Ma & Sun (1982). The two pests occur sympatrically in apple production areas in China ( Liu et al. 2013).
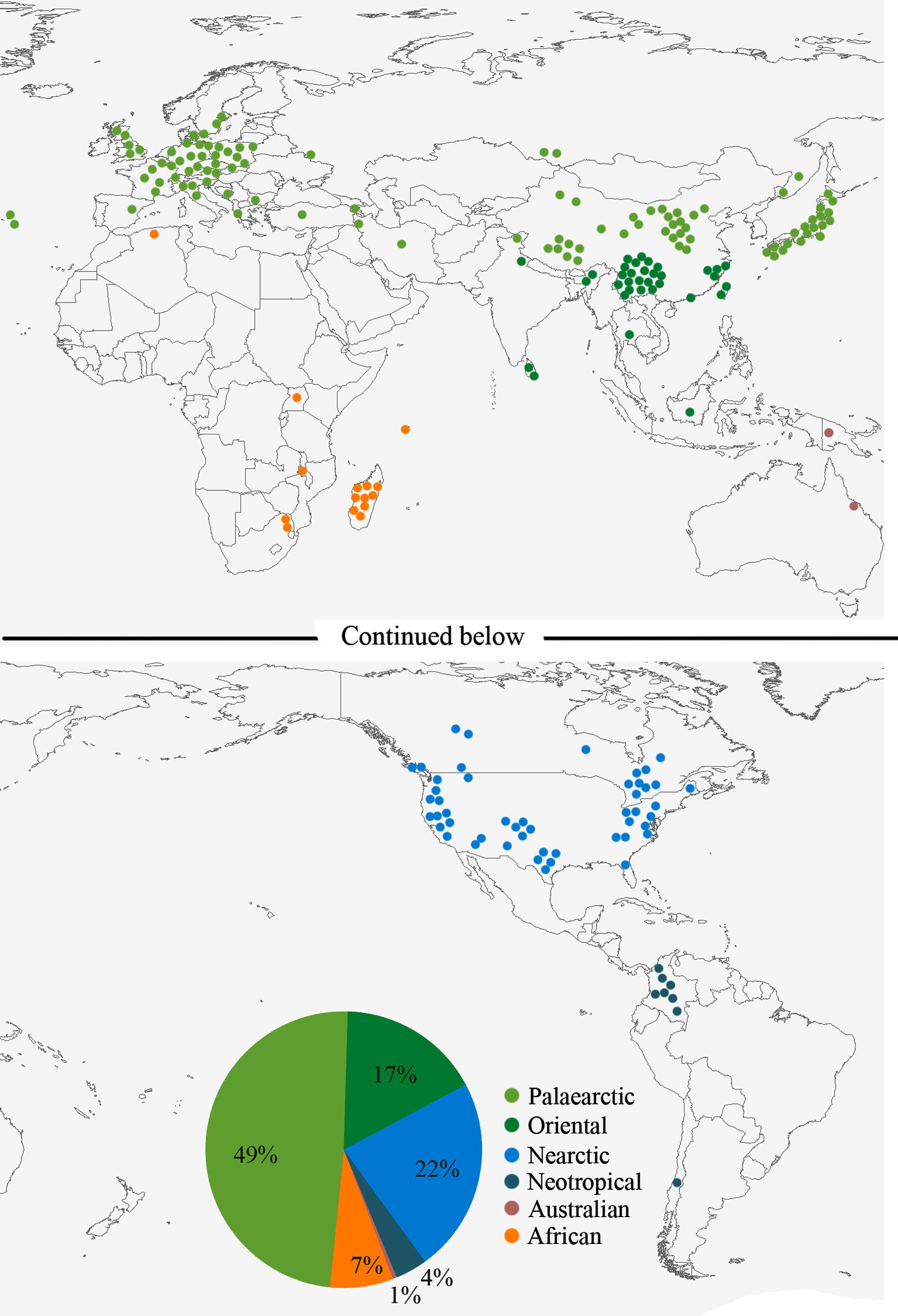
Distribution. Argyresthia is most diverse in the Northern Hemisphere, especially in Europe and North America ( Heppner & Duckworth 1983; Karsholt et al. 2013; Lewis & Sohn 2015), but fundamental concepts of distribution must be modified when considering that the Chinese fauna now includes more than 25% of the world Argyresthia species. The world distribution and biodiversity of Argyresthia is analyzed based on the type localities of all species including the new species described herein ( Fig. 11 View FIGURE 11 and Table 1). Argyresthia species are known from all zoogeographic regions, with the highest species richness in the Palearctic Region (49%), the Nearctic Region (22%), and the Oriental Region (17%). Most Oriental species are found in the northern part, with the highest species diversity in southwest China, mainly in Yunnan Province. The known diversity of the Neotropical Region (4%) and the African Region (7%) were documented chiefly by the works of Zeller (1877) on Colombia and Gibeaux (1983a) on Madagascar. Lowest diversity is found in the Australian Region (1%) with only two species known, one from Australia and the other from New Guinea.
Genus Palearctic Oriental Nearctic Neotropical Australian African Argyresthia 1 0 4 3 4 4 9 8 2 1 6 Individual Argyresthia species are typically restricted to a single zoogeographical region, with a few spanning across two regions. Six species are known from both the Palearctic and the Nearctic regions: A. ( A.) conjugella Zeller, 1839 , A. ( A.) goedartella (Linnaeus, 1758)) , A. ( A.) pygmaeella ([Denis & Schiffermüller], 1775), A. ( B.) cupressella Walsingham, 1890 , A. ( B.) thuiella (Packard, 1871) , and A. ( A.) pruniella (Clerck, 1759) ( Gibeaux 1983b; Agassiz 1996; Agassiz & Tuck 1999; Pohl et al. 2010). Some of these distributions are the result of inadvertent introduction by man. For example, A. ( A.) pruniella was introduced into Europe from North America ( Agassiz 1996). Distribution across two zoogeographical regions is more common in the adjacent Palearctic and Oriental regions, which lies mostly in South China. For example, A. ( A.) angusta , typically known in Kyusyu , Japan, is also found in Guizhou , China, but this species does not penetrate deep into the second region. The distribution of the apple fruit moth A. ( A.) conjugella corresponds to the regions of apple fruit production in China, including Yunnan in the Oriental Region . Thus, this pest occupies at least three zoogeographical regions.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SuperFamily |
Yponomeutoidea |
|
Family |
Argyresthia Hübner
| Liu, Tengteng, Wang, Shuxia & Li, Houhun 2017 |
Paraargyresthia
| Moriuti 1969: 30 |
Eurynome
| Chambers 1875: 304 |
Blastotere
| Ratzeburg 1840: 240 |