Gymnobelideus leadbeateri, McCoy, 1867Petaurus australis, 1791
|
publication ID |
https://doi.org/10.5281/zenodo.6656820 |
|
DOI |
https://doi.org/10.5281/zenodo.6620341 |
|
persistent identifier |
https://treatment.plazi.org/id/038A1613-FFBF-FF94-86E5-FE23C853F561 |
|
treatment provided by |
Felipe |
|
scientific name |
Gymnobelideus leadbeateri Petaurus australis |
| status |
|
6.
Leadbeater’s Possum
Gymnobelideus leadbeateri View in CoL
French: Triok de Leadbeater / German: Hornchenbeutler / Spanish: Falangero de Leadbeater
Other common names: Fairy Possum
Taxonomy. Gymmnobelideus leadbeateri McCoy, 1867 ,
Bass River, Victoria, Australia.
This species is monotypic.
Distribution. C Victoria, SE Australia. View Figure
Descriptive notes. Head-body 15-17 cm, tail 15-18 cm; weight 100-170 g. General appearance is gray to grayish brown above, with prominent dark mid-dorsal strip; ventral surface is a pale cream color. Tail is club-shaped, broader near tip than at base.
Habitat. In the Central Highlands this species occurs at up to 1200 m in forests dominated by myrtle family trees such as mountain ash ( Eucalyptus regnans), shining gum (E. mitens), alpine ash (FE. delegatensis), snow gum (E. pauciflora), and mountain swamp gum (FE. camphora); within these montane ash forests the optimal habitat (containing 1-3-3 ind/ha) is in regrowth 15-50 years old and having a dense understory of acacias and an overstory of more than four large trees (with diameter at breast height greater than 1 m) per hectare. Yellingbo Nature Conservation Reserve includes three linear sections of riparian forest totaling 640 ha, with three main forest types: open forest dominated by messmate (FE. obliqgua), green scentbark (FE. fulgens), and narrow-leafed peppermint (LE. radiata) on dry, well-drained slopes near reserve periphery; and two distinct riparian vegetation types, one being manna gum (E. viminalis) forest with understory of blackwoods ( Acacia melanoxylon, Fabaceae ), and the other mountain swamp gums with, in places, a dense middle story of myrtle family trees such as woolly teatree ( Leptospermum lanigerum), manuka (L. scoparium), prickly tea-tree (L. continentale), swamp paperbark ( Melaleuca ericifolia), or scented paperbark (M. squarrosa).
Food and Feeding. Approximately 20% of the diet of Leadbeater’s Possums comprises invertebrates that are foraged from underneath the shredding bark of eucalypts. The possums eat adult and larval beetles (Coleoptera), flies (Diptera), leathoppers (Hemiptera), ants (Hymenoptera), adult and larval moths (Lepidoptera), tree-crickets (Orthoptera), thrips (Thysanoptera), spiders (Araneomorpha), and pseudoscorpions (Pseudoscorpionida). The remaining 80% of the diet comes from exudates, including those licked from tree trunks ofsilver wattle ( Acacia dealbata) and mountain hickory wattle (A. obligua), and also honeydew (sugary waste excreted by insects) and manna from eucalypts.
Breeding. The estrous cycle is thought to be slightly shorter than 30 days and the gestation period is 15-17 days. If the first litteris lost the animals are able to breed again within the same breeding season. In the highlands young are typically born during one of two annual breeding seasons, April-June or October-December; in the lowland swamps births have been recorded year-round. Females produce litters of one or two (average litter size 1-5); there are four teats. It appears that females need a territory in order to breed and raise young successfully. The young remain in the pouch for 80-93 days and first emerge from the nest at c.110 days. At Cambarville, in the Central Highlands, the sexes differ in age of dispersal, males dispersing at 15 months and females at ten months; this may be to ensure that the offspring do not replace their mothers in the colony, and therefore reduces inbreeding. In contrast, at the Yellingbo Nature Conservation Reserve, both sexes stay within the maternal home range until they are at least 16 months of age. The reproductive life also varies, being 1-6 years at Cambarville and 3-3 years at Yellingbo. Genetic studies of the Yellingbo population revealed that 62% of adult males and 66% of adult females hold the position of “resident breeding individuals” in their colony. Within a colony, breeding vacancies arise following deaths and are quickly filled by immigrants from neighboring colonies; these new breeders are typically more than two years of age.
Activity patterns. Nocturnal. Leadbeater’s Possums emerge from their dens c.16 minutes after dusk. During the day the animals rest up in dens made in hollows of very large, old trees; in the Central Highlands they spend almost 75% of their time in their nests. The possums utilize several dens, which are located 1-30 m above the ground in large communal nests; the nests consist of shredded bark built in the hollow center of a large dead orliving tree.
Movements, Home range and Social organization. These possums live in colonies of 2-12 individuals that consist of a monogamous breeding pair and one or more generations of non-breeding offspring. The size of the colony appears to vary according to habitat quality. Membership of the colony is maintained by scent marking each other and by mutual tail-licking; these family groups defend resources and huddle together when conditions are cold. In the mountain ash forests, dispersing animals regularly visit established colonies and membership turnover is high. A colony utilizes a home range of 1-2 ha that is actively defended from members of adjacent colonies. Most animals use two or more trees, and the distance between nesting trees usually exceeds 50 m. Possible reasons for the den-swapping behavior include attempts to relieve the burden of ectoparasites, to reduce the risk of predation, or both. Leadbeater’s Possums make several calls, which appear mostly to aid in communication between individuals of the colony and potentially to mark their territorial boundaries; these include a high-pitched staccato call that is often made when fighting, particularly during territorial disputes between neighboring colonies, and a type of hiss that is used when alarmed, as when fighting or threatened by a predator. Main predators of Leadbeater’s Possums appear to be greater sooty-owls (7yto tenebricosa), Australian masked-owls (7. novaehollandiae), powerful owls (Ninox strenua), and possibly Spottedtailed Quolls (Dasyurus maculatus), as well as introduced species such as domestic/feral cats (Felis catus), domestic/feral dogs, and Red Foxes (Vulpes vulpes).
Status and Conservation. Classified as Endangered on The IUCN Red List. Following intense fires in 1939, this species, already extremely rare, was then believed extinct. In 1961, however, it was rediscovered near Marysville, and was believed at that time to be restricted to Central Highlands of Victoria, but in 1989 an isolated population was found in a patch of habitat at Yellingbo Nature Conservation Reserve, some 17 km away. In the early 1990s, the Yellingbo population was thought to number ¢.200 individuals and the main Central Highlands population ¢.2000 mature individuals. At that time a decline of ¢.90% over the following 30 years was predicted owing to loss of den trees and suitable nesting habitat. More recently, the Yellingbo Reserve is thought to sustain 80-100 individuals. Historically, this species appears to have had a wider distribution in Australia, as evidenced by Pleistocene fossil deposits in southern New South Wales (Wombeyan Caves and Marble Arch) and in eastern Victoria (Buchan). There are also five specimens collected between 1867 and 1910 within Victoria from Mt Wills, Koo-wee-Rup, and Bass River; further, anecdotal reports have been recorded from Dartmouth, Wonnangatta, Castle Hill, Strzelecki Ranges, and Black Forest. The contraction in the species’ distribution provides good evidence of the changes in climate and vegetation structure over the last 30,000 years or more. The fossil record suggests that these possums were relatively common in wet sclerophyll forests, whereas they were rare or absent in dry sclerophyll forests. Wildfires that spread through the Central Highlands in 1939 resulted in a loss of tree hollows throughout the species’ range. These trees continue to collapse, and new hollows will not appear within the regrowth forests until ¢.2075; a dramatic decline of ¢.90% in possum numbers by 2020 is predicted. Some authors have proposed the erection of nestboxes in order to ameliorate the imminent cavity shortage, but research in the Central Highlands suggests that these have a low rate of occupancy and would be ineffective as a large-scale solution. Given the very low levels of nestbox occupancy, tree-harvesting regimes that retain hollows are needed; logging practices are presently not ecologically sustainable, and modified forestry practices need to be adopted. In contrast, nestbox-occupancy rates at the Yellingbo Nature Conservation Reserve (where there are few tree hollows) are much higher, Leadbeater’s Possums utilizing 75% of the 150 boxes supplied. The discrepancy in nestbox usage between the two locations may be due to there being other nesting opportunities within trees in the Central Highlands, so that, as these trees continue to collapse or are harvested, the use of nextboxesin that region may increase. In addition to the loss of tree hollows through natural sources, more than 75% of the forests within the species’ known distribution are designated for harvesting by the timber and paper industries; with clear-cutting, virtually all trees on a coupe of 15-40 ha are removed in a single operation. As the rotation cycle is 50-80 years,this is not long enough for hollows to develop in regrown trees before these, too, are harvested. As this species has such a limited distribution, conserving it places it in direct confrontation with the timber industry. Clear-cutting impacts the vegetation structure of forests, affecting availability of nest trees, availability of forest sites for the species, and the arrangement of suitable patches in the forest landscape. Two eminent scientists (one of whom has studied this species over 25 years) argued that recent government policies in Victoria run counter to the conservation of this species and appear to be driving it to extinction, even though it is the faunal emblem of that State: they assert that, after a system of protected areas was established in 2008, the government of Victoria sanctioned changes to the legislation and substantially watered down protocols for habitat protection for this species; logging of “protected” areas has been permitted, allowing the destruction of known habitat of LLeadbeater’s Possum, which will render these areas unsuitable for at least 150 years.
8.
Yellow-bellied Glider Petaurus australis View in CoL
French: Possum a ventre jaune / German: Grofser Gleithornchenbeutler / Spanish: Falangero planeador de vientre amarillo
Other common names: Bellied Glider, Fluffy Glider
Taxonomy. Petaurus australis Shaw, 1791 View in CoL , Sydney, New South Wales, Australia.
Although the geographically isolated population in north-east Queensland has been described as a separate race (reginae) named by O. Thomas in 1923, no subspecies are currently recognized. Monotypic.
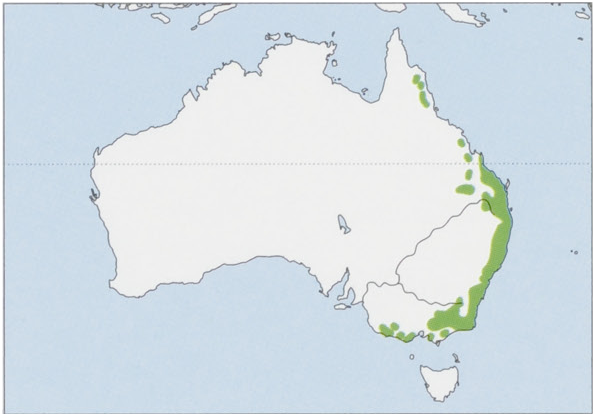
Distribution. E Australia in NE Queensland (between Mt Windsor and Yamanie, on bank of Herbert River Gorge), and patchily from EC Queensland S along coast through New South Wales to the Victoria—South Australia border. View Figure
Bibliography. Brazenor (1962), Flannery (1994a), Hansen & Taylor (2008), Hansen etal. (2009), Harley (20044, 2004b), Harley & Lill (2007), Harley et al. (2005), Lindenmayer (1996), Lindenmayer & Meggs (1996), Lindenmayer & Possingham (2013), Lindenmayer, MacGregor & Gibbons (2002), Loyn & McNabb (1982), Menkhorst (2008b), Smales (1994), Smith, A.P (1984a, 1984b), Smith, A.P & Harley (2008), Smith, A.P & Lindenmayer (1988), Wilkinson (1961).
Descriptive notes. Head—body 24-31 cm,tail 38-47 cm; weight 470-725 g (males) and 435-660 g (females). Conforms to Bergmann’s Rule: individuals in more tropical regions have a smaller body mass than those from more temperate regions. This species is the largest petaurid glider. It is gray brown (sometimes rich brown) above, with a distinctive black dorsal stripe extending from head to rump. Young animals typically have a white belly, and adults have a distinctive yellow belly. The speciesis also the most vocal of the petaurid possums,so is easily distinguished from other petaurid gliders.
Habitat. Inhabits wet sclerophyll forest, open coastal forest, and foothill forests that are dominated by mature, tall eucalypts. One study documented a definite preference for forest associations that contain gum-barked and winter-flowering species. Within these associations, the abundance of animals was correlated with microhabitat variables and structural variables representing forest age and degree of disturbance; significant factors included the structural variable,site productivity, and the number of dead hollowbearing trees (which relate to the foraging and denning requirements of the glider).
7.
Northern Glider
Food and Feeding. The diet ofthis glider consists primarily of insects, arthropods, and exudates, including sap from trees, lerps, manna, honeydew, and nectar and pollen, the proportion of each varying among different locations. Insects are often caught while the glideris foraging through peeling bark. When sap-feeding,it uses its lower incisors to gouge into a Eucalyptus , Corymbia (both Myrtaceae ), or sometimes Acacia (Fabaceae) tree (referred to as sap-site trees), after which the sap can continue to flow and be eaten by the gliders for several decades. The incisions made form a V-shape that is typically c¢.12 cm long and has an angle of approximately 100°; they are cut through the outer bark, inner bark, and cambium layers of the trunk and form a neat channel typically 5-10 mm wide and 4-10 mm deep,just touching the sapwood, which is not incised. The incised trees typically have a minimum diameter at breast height greater than 40 cm. Some tree species extensively used at one location may be completely ignored at another. Tree sap is particularly important at extremes of the range, including north Queensland and western Victoria, as it accounts for more than 80% of the diet. In other parts of the glider’s distribution sap constitutes ¢.30% of the diet. Typically, only one or two species of tree are used at a particularsite, even though five or six may be available, and fewer than 1% of possible trees are incised for sap at any one location. Individuals also use fewer than ten trees of a particular species and on average use only two trees at a time, of which they choose a single one and feed on sap for the whole night. In north Queensland, only one tree species, the red mahogany ( Eucalyptus resinifera), is used. Variation in sap flow appears to occur independently of rainfall or microhabitat where the tree is located. Experimental incising oftrees, designed to mimic the effects of feeding by gliders, have failed to show any effect on sap flow, which suggests that the incidence of sap-feeding is determined by a tree’s pattern of sap flow, and that there may be trees with unusual patterns of sap flow that the gliders select as the most favorable trees to incise. Nectar and pollen are also very important to Yellow-bellied Gliders, particularly in southern New South Wales, where gliders may visit a single flowering tree (possibly all night). The availability of nectar and pollen varies temporally and, at times when few flowering trees are present,gliders may choose trees with fewer flowers than at times when flowering trees are abundant. At other times, when flowering trees are superabundant or scarce, there may be no relationship between the number of flowers in a tree and the duration ofvisits by gliders. In one study, at intermediate levels of abundance, the amount of time that a glider spent in a tree was related to the number of flowers in a tree.
French: Possum de Ziegler / German: Nordlicher Gleithdrchenbeutler / Spanish: Falangero planeador septentrional
Taxonomy. Petaurus abidi Ziegler, 1981 View in CoL ,
“Papua New Guinea, West Sepik Province, Mount Somoro (also spelled Somero; approximate coordinates 3°25’S, 142°05°E), about 1220 m, approximately 9-7 km ENE Lumi (Patrol Post).”
This species is monotypic.
Distribution. Mt Somoro and adjacent areas in Torricelli Mts, NW Papua New Guinea. View Figure
Descriptive notes. Head—body 25-28 cm,tail 35-38 cm; weight 228-332 g. This species can be readily distinguished from the New Guinean Sugar Glider (P. breviceps), which occurs in same area, by its much greater size and predominantly black tail. It differs from the three Australian gliders in its very long, thickly furred, yet not bushytail. Its dorsal fur is pale gray, washed with yellowish brown, especially toward midline of back and on rump.
Habitat. Occurs in primary, mid-montane tropical moist forests, and in rural gar dens close to forest. Found at elevations above 300 m, but thoughtto be rare below 800 m; most are recorded between 800 m and 1200 m.
Food and Feeding. Not much is known of the diet. Specimens have been collected while they were feeding on figs ( Ficus , Moraceae ), and captive animals have thrived on guavas, bananas, and Syzygium (Myrtaceae) fruit.
Breeding. Little is known about the reproduction of the Northern Glider. A lactating female was collected in March; another female was carrying a pouch young that was just acquiring fur on the head and weighed 6-5 g.
Activity patterns. There is no information available for this species.
Movements, Home range and Social organization. The ecology of this petaurid has not been studied well to date, but some anecdotal observations exist. Three adult males and two adult females were found in a hollow, which had been enlarged by chewing, in an isolated senescent tree (known locally as “diri”). Captive individuals have been heard to make a series of loud growls and shrieks.
Status and Conservation. Classified as Critically Endangered on The IUCN Red Last. This New Guinean species is known only from Mount Somoro and nearby areas in the Torricelli Mountains. Its extent of occurrence is limited, covering less than 100 km?, and because all individuals are in a single location, because the extent and quality of its habitat are continuing to decline owing to heavy deforestation and conversion to gardens, and because it is hunted for food, it is considered to be at the highest risk. Only seven animals have been captured in the last 20 years. In the future the Northern Glider may be found to occur in the poorly surveyed North Coastal Ranges of Papua, which comprise a section of mountain range c.100 km in length and a few tens of kilometers wide, from at least Fas 2 Village (to the east of Mount Menawa) in the west to Mount Sapua in the east. For the present, however,it is essential that it be afforded protection in conservation areas.
Bibliography. Flannery (1994a), Leary, Wright, Hamilton, Singadan, Menzies, Bonaccorso, Salas et al. (2008a), Ziegler (1981).
Breeding. Females typically give birth to a single young annually, although there are occasional records of two young. Births peak during the months ofJuly-October. There appears to be a general trend toward slight regional differences in breeding times, populations in Queensland breeding mostly between June and late August, those in southern New South Wales during February-April, and those in Victoria from August to December. The young reach sexual maturity at approximately 15-24 months of age.
Activity patterns. Yellow-bellied Gliders are nocturnal, emerging from their dens c.19 minutes after dusk. When not in their tree-hollow dens they spend ¢.90% of the time in foraging-related activities, including climbing, gliding, and feeding, during which they can traverse more than 2 km per night. They sometimes return to the nest hollow after dawn during the shorter nights of summer. Gliders spend most of the night outside the dens (96% in summer, 73% in winter), and devote an average of 81% of this time to feeding; they are inactive for only 2% of the time. When feeding time is coupled with that for other behaviors essential for foraging (i.e. gliding and climbing), 90% of the time outside the den is accounted for. This is among the highest values yet found for a mammal. Various studies showed that the amount of time gliders spent in trees was significantly greater when feeding on exudates (on average, more than 48 minutes per tree) than when harvesting arthropods and other food types from under loose bark (less than 13 minutes per tree); distances traversed by gliders observed for entire nights (range 590-2350 m) appear to reflect this pattern, but further data are required. Glides averaged 40 m, but ranged up to 100 m; and type of food influenced the number of gliders feeding together in the same tree.
Movements, Home range and Social organization. These gliders typically live in family groups, in home ranges of 30-65 ha, the largest of any petaurid. These home ranges are defended; gliders that are not part of the group are attacked by the resident animals and occasionally killed. The home range appears to be maintained by scent marking branches with the frontal gland and chest gland. As a result of the large, mutually exclusive home ranges, Yellow-bellied Gliders have a density of only 0-10-0-19 ind/ ha in preferred habitat and as low as 0-04 ind/ha in other habitat. Within their home range they have several dens that they often share with each other. In traversing their home range these gliders make glides of approximately 40 m, with glides over 140 m recorded. A family group contains two to six individuals, consisting of an adult male with one or more females and their offspring. Thesize of the groups and the apparent mating system appear to depend on the quality of the habitat and the associated availability of food. In some populations, smaller group size is preferred because flowering resources are more limited, and a monogamous mating system predominates, with one pair and its offspring in groups containing 2-3 individuals; in other populations, with access to a combination of eucalypt species that provides abundant food resources throughout the year, larger polygynous groups of up to six individuals (one adult male and two adult females and their offspring) occur. Within the groups cohesion appears to be maintained through scent marking. Adult males have scent glands on the head, chest, and near the base of the tail, and members of the group rub themselves against these glands. This species is the most vocal of all Australian gliders, with an extraordinary 17 differentcalls recorded, including loud shrieks, long gurgling calls, and soft buccal clicks. The rate of calling varies through the night, being more frequent in the first three hours of activity after emergence, with calling as often as 10-15 times per hour while foraging. The loud shrieks can be heard 500 m away. A moan and a gurgle are emitted only during gliding, suggesting that these serve as a method for coordinating the movements of individuals within the group, and may also serve a territorial function. Calling frequency increases near the boundary of the home range. Studies show call rates of nine times per 30 minutes near the perimeter, whereas at the core of the home range only two calls per 30 minutes were recorded. The territorial nature of these vocalizations is supported by experimental playbacks of the calls used to signal an intruder: calling rates by the gliders increased significantly, to six calls per 15 minutes, compared with only 2-8 calls per 145 minutes before the playback.
Status and Conservation. Classified as Least Concern on The IUCN Red List. The population in north Queensland (possibly a separate subspecies) is considered to be vulnerable and the one in South Australia is considered endangered. These two populations, at the extremes of the range, are isolated from the remainder. Although this species has a wide distribution with a presumed large population, occurs in a number of protected areas, and is unlikely to be declining at a rate that would give cause for concern, it is patchily distributed, and all available information on the natural history of this glider indicates that it is particularly sensitive to habitat-clearing and fragmentation. Where habitat is protected from clear-cutting, degradation may become a major problem as a result offires and extreme weather events such as high winds that blow trees over. Timber-production areas need to be managed appropriately to ensure that large areas of intact, contiguous forest are retained, with particular focus on maintaining tree hollows and sap trees. From a study of habitat preferences in south-east Queensland, implications for forest management include the need to retain both mature gum-barked eucalypt species and live hollow-bearing trees during harvesting operations. Long-term conservation of the glider is compatible with low-intensity selective logging, where a high proportion of ironbark and gum-barked stems with a diameter at breast height greater than 50 cm is retained during logging events. The recruitment of potential hollow-bearing trees will be essential, and dead hollow-bearing trees will need to be maintained to provide an interim hollow resource. A population-viability analysis of the Yellow-bellied Glider proposed that 150 groups of gliders were needed to support a viable population, and thatthis required a minimum of 9750 ha where all habitat is used. As no more than 28-54% of the available habitatis exploited in some populations,it was suggested that 18,000-35,000 ha should be provided as a minimum area to maximize the chances of the species’ long-term survival.
Bibliography. Brown et al. (2007), Carthew (2004), Craig (1985, 1986), Eyre & Goldingay (2003, 2005), Eyre & Smith (1997), Flannery (1994a), Goldingay (1986, 1987, 1989, 1990, 1991, 1992, 1994, 2000a, 2008), Goldingay & Kavanagh (1990, 1991, 1993), Goldingay & Quin (2004), Goldingay et al. (2001), Henry & Craig (1984), Kavanagh & Rohan-Jones (1982), Mackowski (1988), Menkhorst, Winter et al. (2008), Quin, Goldingay et al. (1996), Russell (1984), Thomas (1923c).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
SubClass |
Metatheria |
|
Order |
|
|
SubOrder |
Phalangeriformes |
|
SuperFamily |
Petauroidea |
|
Family |
|
|
Genus |
Gymnobelideus leadbeateri Petaurus australis
| Russell A. Mittermeier & Don E. Wilson 2015 |
Petaurus abidi
| Ziegler 1981 |
Gymmnobelideus leadbeateri
| McCoy 1867 |
Petaurus australis
| Shaw 1791 |