Deinocroton, Enrique Peñalver & Antonio Arillo & Xavier Delclòs & David Peris & David A. Grimaldi & Scott R. Anderson & Paul C. Nascimbene & Ricardo Pérez-de la Fuente, 2017
|
publication ID |
https://doi.org/ 10.1038/s41467-017-01550-z |
|
DOI |
https://doi.org/10.5281/zenodo.6033673 |
|
persistent identifier |
https://treatment.plazi.org/id/038A8794-FFD2-FFA7-AF6E-FD72C6B3F9C4 |
|
treatment provided by |
Plazi |
|
scientific name |
Deinocroton |
| status |
gen. nov. |
Deinocrotonidae Peñalver, Arillo, Anderson and Pérez-de
la Fuente fam. nov.
Type genus. Deinocroton gen. nov. Monotypic.
Etymology. From Greek deinos, “ terrible ”, and krotÓn, “ tick ”. Gender: neutral.
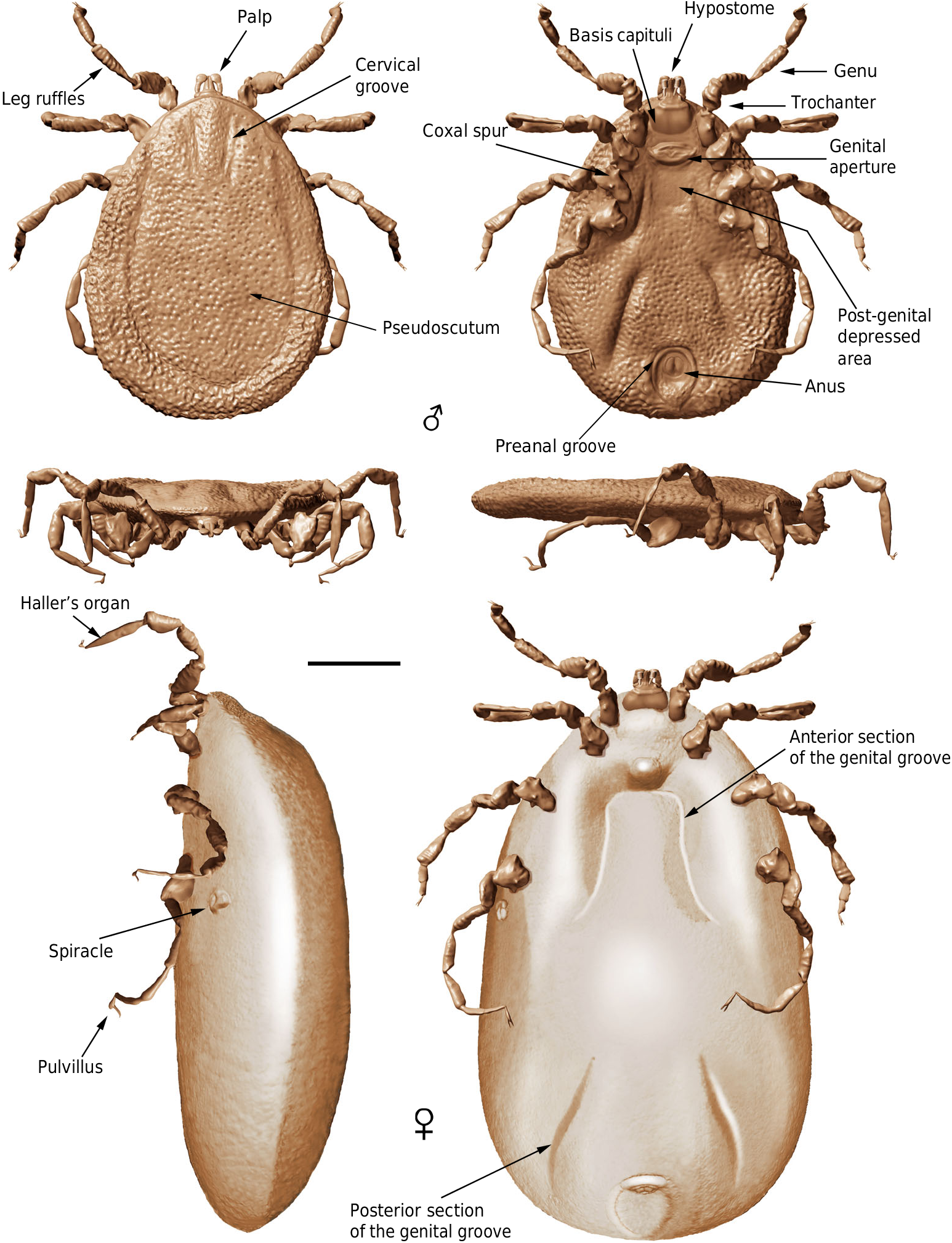
Diagnosis (both sexes). Integument with closely spaced, deep pits, and mound-like elevations between pits; integument not convoluted, lacking microsculpture. Pseudoscutum distinct (abbreviated in females), pitted but without elevations. Eyes absent. Hypostome subterminal. Basis capituli not bordered by coxae I. Palpi elongated, gracile; palpomere II distally thickened and bent in ventral direction, palpomeres III and IV elongated, tubular, fully mobile. Genital aperture transverse, close to the capitulum in males and slightly posteriad in females. Presence of a conspicuous anteroventral depressed area, post-genital in position. Spiracles smooth, medium sized, located at the level of coxae IV. Genital groove distinct, medially divided in two sections and extending posteriorly. Anal pore terminal. Preanal groove prolonged posteriorly, with sides closing. Legs ruffled. All coxae with short spurs in rows. Leg joints not of the ball and socket type but notch-like processes present. Haller ’ s organ proximal capsule completely open. Festoons absent.
Deinocroton draculi Peñalver, Arillo, Anderson and Pérez- de la Fuente gen. et sp. nov.
Etymology. Patronym for the main character of the gothic horror novel by Irish writer Abraham “ Bram ” Stoker, which is a fictionalised account of Vlad III, or Vlad Dracula (ca. 1429 – 1476).
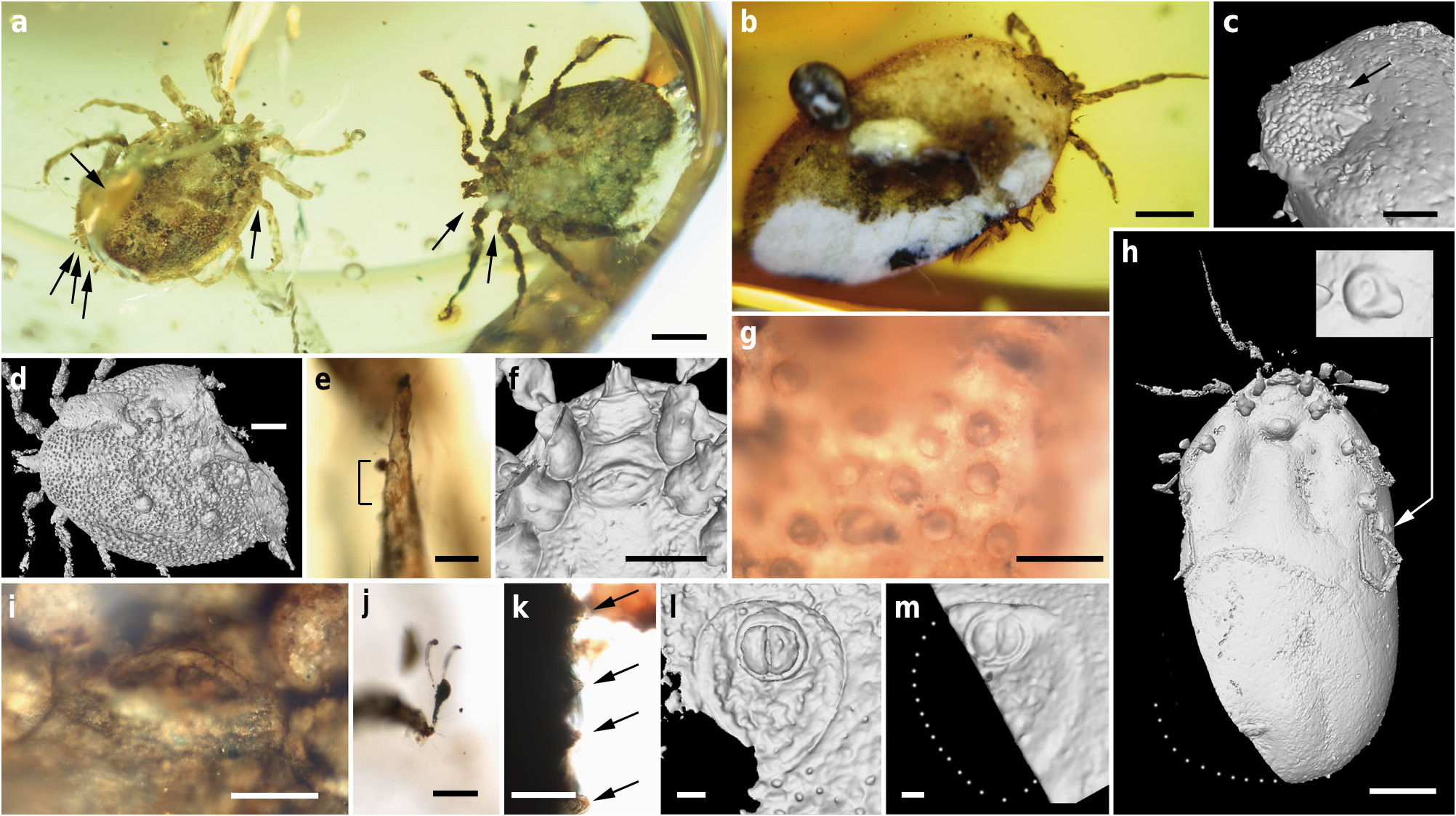
Holotype. Adult male ( AMNH Bu-SA 5 a), ca. 3.9 mm long from posterior margin to apex of hypostome ( Figs. 3 a, e – g, j, k View Fig. 3 , 4 a, f – h View Fig. 4 , 5 a, c, d, f, g View Fig. 5 ; Supplementary Fig. 2 c, e View Fig.2 ).
Additional material. Allotype: female (CM 63007) ( Fig. 4 b, c View Fig. 4 ; Supplementary Figs. 2 d View Fig.2 , 3 View Fig. 3 ). Paratypes: male ( AMNH Bu- SA 5 b) ( Figs. 3 a, d, i, l View Fig. 3 , 4 d, e View Fig. 4 , 5 b, e View Fig. 5 ; Supplementary Fig. 2 b View Fig.2 ) and engorged female (CM 63001) ( Figs. 3 b, c, h, m View Fig. 3 , 5 h, i View Fig. 5 ). All adults (see Supplementary Note 1 for more details). Locality and horizon. Southwest of Tanai (close to Maingkhwan village) in the Hukawng Basin, Kachin State area (northern Myanmar), likely from the Noije bum opencast system of mines; earliest Cenomanian7.
Diagnosis for genus and species. As for the family.
Description. See Supplementary Note 2 for body measurements.
Male: Body outline subcircular. Integument surface with closely spaced, deep pits and with single, mound-like elevations between pits ( Figs. 3 d, k View Fig. 3 , 5 a – c, e View Fig. 5 ), as in females ( Fig. 4 c View Fig. 4 ). Integument not convoluted (cf. Nuttalliella ), lacking microsculpture (e.g., granulations). Body without conspicuous setal vestiture, except setae present on palpi, legs and anal valves, and very sparse setae present on dorsal and ventral integument. Integumentary pits lacking any associated setae.
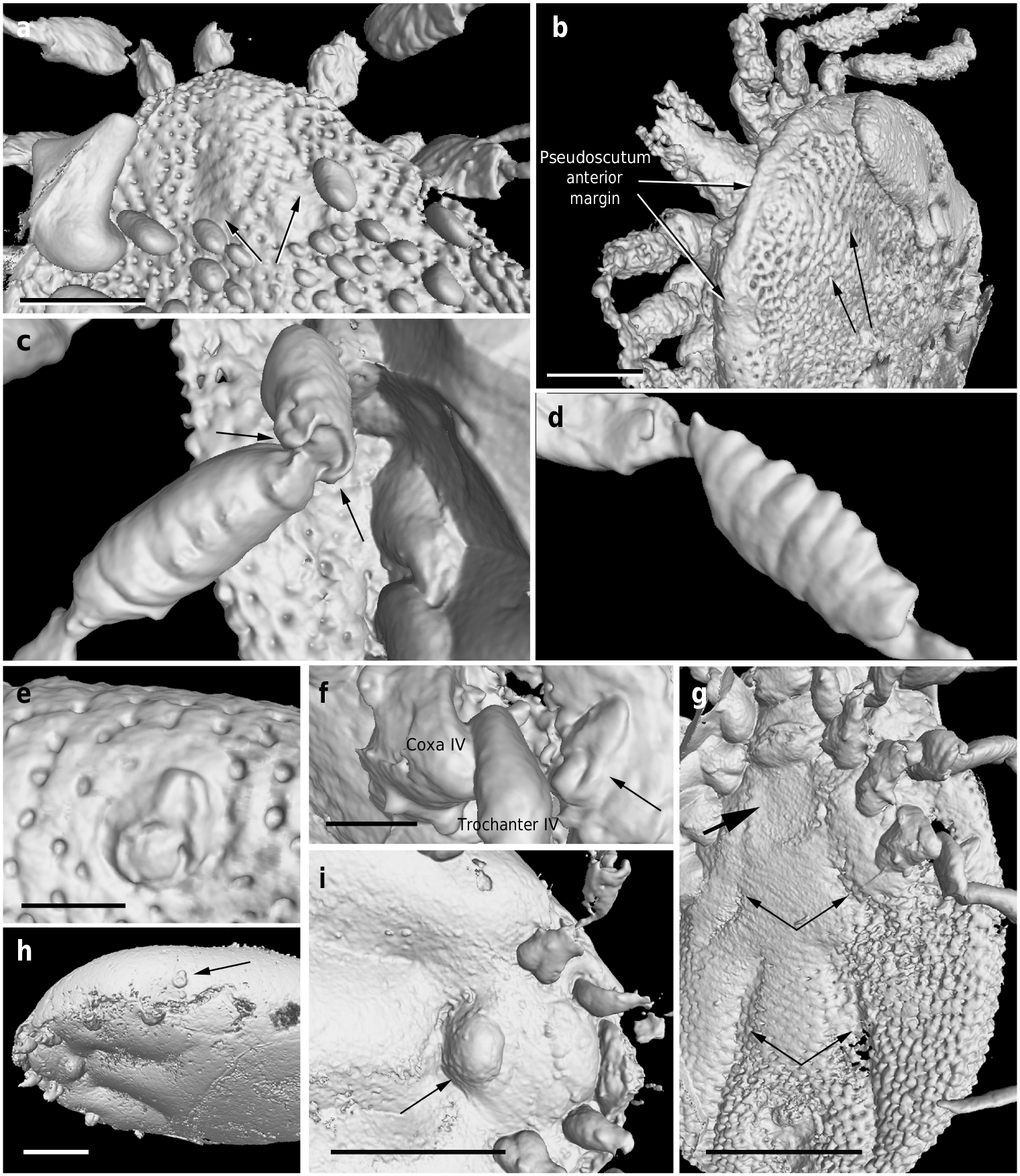
Dorsum. Pseudoscutum distinct (not highly chitinised as in Ixodidae , with integument resembling the rest of body), occupying most part of dorsum, reaching anterior margin of dorsum ( Fig. 3 d View Fig. 3 ), with anterolateral margin broadened posteriorly ( Fig. 5 b View Fig. 5 ). Cervical grooves present, relatively shallow ( Fig. 5 a, b View Fig. 5 ). Pseudoscutum integument with closely spaced, deep pits, but without mound-like elevations as in the rest of body, rendering a surface with smooth appearance in which pits are very apparent ( Fig. 3 g View Fig. 3 ). Pits separated by a length equal to their diameter or less. Festoons absent. Eyes absent.
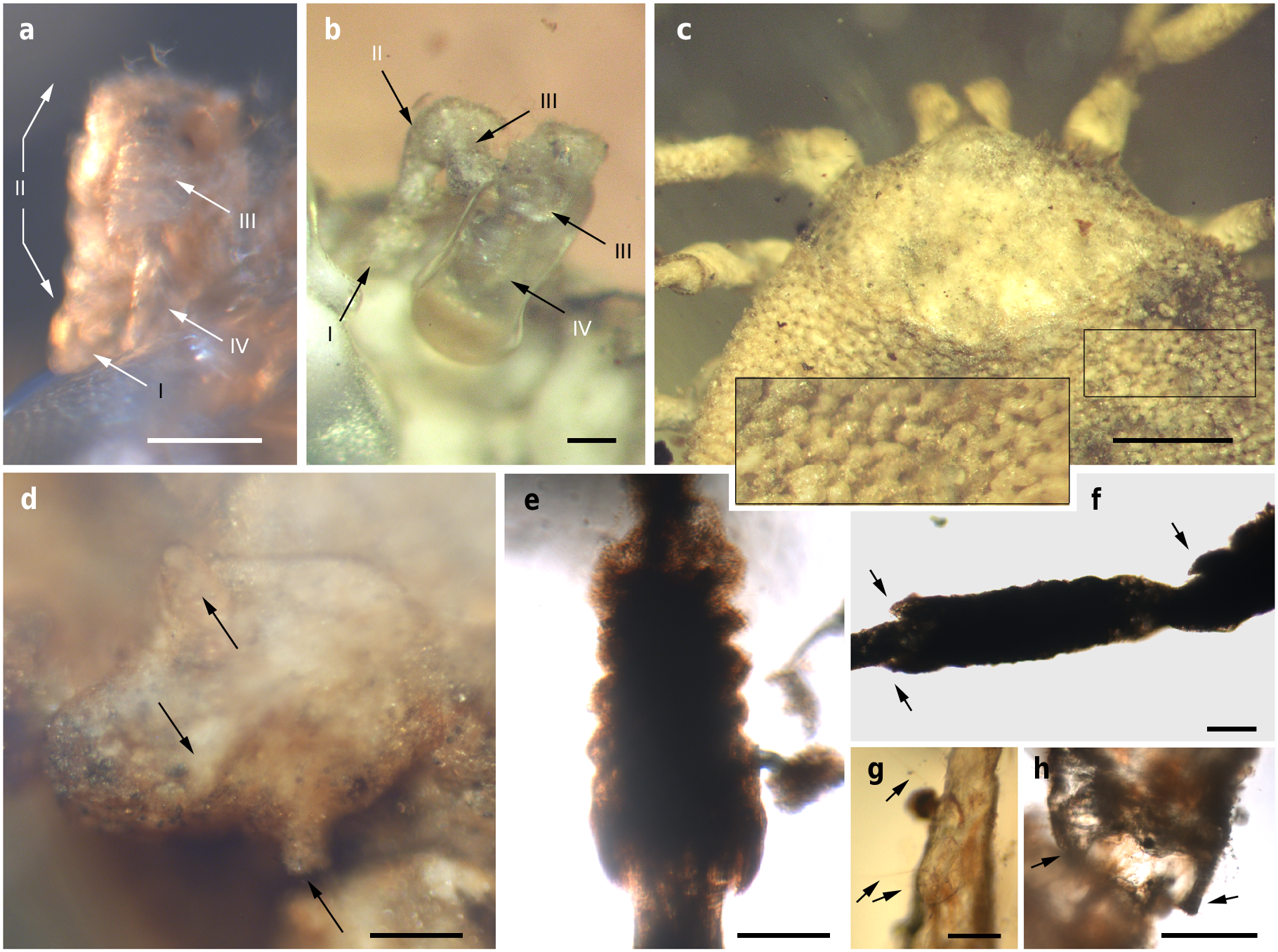
Venter. Capitulum partially visible in dorsal view. Hypostome subterminal (sensu Mans et al. 16) ( Figs. 3 a, d, f View Fig. 3 , 5 b View Fig. 5 ), well developed, reaching apex of palpomere II. Hypostome ultrastructure obscure, dental formula indeterminate. Chelicerae only partially visible in the paratype male. Palpi elongated, gracile (around two times the length of hypostome), fully mobile ( Fig. 4 a View Fig. 4 ; Supplementary Fig. 2 b – d View Fig.2 ), as in females ( Fig. 4 b View Fig. 4 ). Palpomere I short. Palpomere II the longest, distally thickened in width and height, bent distally in ventral direction (creating a ventral concavity, with surface of articulation with palpomere III facing that direction). Palpomeres III and IV elongated, tubular, tapering basally. Palpomere III about two times as long as wide, with surface slightly ruffled. Palpomere IV in terminal position, about four times as long as wide. Palpi without spurs but bearing abundant, fine setae. Basis capituli not bordered by coxae I, with anterior margin rimmed and surface smooth ( Fig. 3 f View Fig. 3 ; Supplementary Fig. 2 b View Fig.2 ); auriculae, cornua and porose areas absent.
Genital aperture a transverse slit in an oval area between anterior half of coxae II ( Fig. 3 f, i View Fig. 3 ), close to capitulum. Presence of a conspicuous anteroventral depressed area ( Fig. 5 g View Fig. 5 ) that is quadrangular in shape and post-genital, laterally limited by anterior section of genital groove. Genital groove well developed and extending posteriorly; medially divided (immediately after coxae IV) into two sections ( Fig. 5 g View Fig. 5 ). Anterior genital groove section extending from coxae II to IV, briefly bordering coxae IV distally (i.e., diverging towards body margin). Posterior genital groove section the longest, grooves progressively diverging posteriorly, slightly bordering anal plate. Spiracle well developed and very close to body margin at level of coxae IV, smaller than in Ixodidae (and in a different position) and larger than in Nuttalliella namaqua 17. Spiracle plate structure sub-triangular in shape and consisting of a small macula and a smooth triangular plate, not fenestrated but bearing two small concavities ( Fig. 5 e, f View Fig. 5 ), as in females ( Figs. 3 h View Fig. 3 , 5 h View Fig. 5 ); macula projecting towards ostium to form a lip; entire plate arising from a depressed cuticular area. Preanal groove prolonged posteriorly, with sides closing, delimiting a guitar pick-shaped anal plate ( Fig. 3 l View Fig. 3 ), as in females ( Fig. 3 m View Fig. 3 ). Anal pore close to posterior margin of body. Anal valves with a few long and fine setae.
Legs. Long and strongly flattened laterally from trochanters to tarsi; arising within anterior two-fifths of total body length. Leg joints not of ball and socket type as in Nuttalliella , but leg articles with paired, notch-like ventrodistal processes (without forming sockets for the articulation, balls not distinct), more apparent in basal articulations ( Figs. 4 f, h View Fig. 4 , 5 c, d View Fig. 5 ). Slight separation between coxae, except coxa I contiguous with II. Coxae armed with rows of small, shallow spurs (i.e., rounded tubercles, such as in some ixodids and Nuttalliella ) ( Figs. 3 f View Fig. 3 , 4 d View Fig. 4 , 5 f View Fig. 5 ): one spur on coxa I — in medioposterior position — and three on each coxa II, III, and IV. Three coxal spurs forming a row in coxa II, with two of them in a medial, posterior position while third one in a distal, anterior position. Three coxal spurs aligned in medial position in coxae III and IV (two close together in a slightly basal, posterior position and third one in anterior position at middle of coxa). Trochanter without spurs. Femur, genu, and tibia bearing a sculptured surface of transverse ridges (ruffles), especially marked in genu ( Figs. 4 e View Fig. 4 , 5 d View Fig. 5 ). Trochanters I and II with very shallow ruffles, almost indistinct. First pair of legs with deeper ruffles. Femora I and II positioned very high and strongly flattened laterally. Femur III flattened laterally and high only basally. Femur IV tubular. Haller ’ s organ conspicuous; although only observed in right tarsus I of holotype ( Figs. 3 e View Fig. 3 , 4 g View Fig. 4 ; Supplementary Fig. 2 e View Fig.2 ) due to preservation of remaining specimens, situated on a dorsal elevation of tarsus I and composed of two parts, a completely open (without a transverse slit) proximal capsule having long setae and a distal pit followed by more long, distinct setae, capsule larger than pit. Basitarsus as long as tarsus in legs II – IV. Pretarsi with two curved pretarsal claws and abundant, long setae. Pretarsal claws large. Pulvilli poorly developed ( Fig. 3 j View Fig. 3 ).
Female: As in male with the following exceptions: Integument, including that of pseudoscutum, with pits not as well defined as in males. Pseudoscutum abbreviated ( Figs. 3 c View Fig. 3 , 4 c View Fig. 4 ; Supplementary Fig. 3 View Fig. 3 ), occupying the anteriormost part of dorsum. Genital aperture in a more posterior position than in males, between coxae II and III, and apparently showing a smooth surface (Supplementary Fig. 3 View Fig. 3 ). Marginal groove absent.
Remarks. A suite of unique, presumably derived characters defines Deinocrotonidae: the integument structure, the palp morphology, and the shape of the preanal groove. Likewise, the discontinuous genital groove is unique among ticks. The subterminal hypostome and the presence of a pseudoscutum suggest a close relationship between Deinocrotonidae and Nuttalliellidae . Pending a phylogenetic analysis when more material is available (see Supplementary Note 3), we propose here that both families are sister to ( Ixodida + Argasidae ). So far, a few more deinocrotonids have been found in Burmese amber, and one additional undescribed immature specimen from 105 Ma old Spanish amber most likely belongs to this new family. Apart from the unique characters among ticks, the new family differs from Nuttalliellidae in the following features (see Supplementary Tables 1 and 2): ( 1) pseudoscutum pitted (vs. mesh-like), ( 2) pseudoscutum reaching the anterior margin of the dorsum in males, ( 3) cervical grooves present, ( 4) capitulum not bordered laterally by coxae I, ( 5) basis capituli simple and with smooth surface, ( 6) cornua absent, ( 7) genital area smooth (vs. irregularly striated), ( 8) anteroventral depressed area in post-genital position (vs. in pre-genital position), ( 9) all coxae armed and spurs forming rows, ( 10) leg joints not of the ball and socket type, at least as in Nuttalliella , ( 11) proximal capsule of Haller ’ s organ completely open, ( 12) different morphology and size of the spiracle, and ( 13) preanal groove different in microscopic detail (smooth vs. posterior and anterior margins with dentate integumental projections).
The pseudoscutum in Deinocrotonidae occupies most of the dorsum in males and is abbreviated in females, as occurs in ticks with a scutum/pseudoscutum. The special shape of palpomere II, distally thickened and bending distally in a ventral direction ( Fig. 4 a, b View Fig. 4 ; Supplementary Fig. 2 b – d View Fig.2 ), appears to be an adaptation to protect the distal part of the gnathosoma dorsally and anteriorly, especially the delicate teeth of the hypostome and the chelicerae. Such expansion of the distal part of the palpomere II is present in all ixodids (namely their upper inner margin, creating an inner groove), although palpomere III is also expanded, taking part in the protection of the gnathosoma, and both palpomeres are straight, directed forwards18, 19. In Deinocroton , palpomere III is elongated and tubular, directed ventrally due to the surface of articulation between palpomeres II and III facing that direction and due to the shape of the palpomere II. In Nuttalliella , palpomere II is massive, expanded laterally and provides most of the gnathosomal protection; palpomere III is smaller, triangular in shape and slightly laterally expanded ventrally, whereas both palpomeres are straight, directed forwards as in ixodids20. Argasids lack any palpomere expansion for gnathosomal protection due to the ventral position of their capitulum in adults. On the other hand, the Haller ’ s organ in deinocrotonids has a generalised morphology, with a proximal capsule and a distal small pit, but fine details are obscure under optical microscopy and they have remained unresolved using CT-scanning. Nevertheless, the proximal capsule is fully open (lacking a transverse slit) as in Ixodes , and unlike in other ixodids, argasids and Nuttalliella 21 – 23. Furthermore, CT-scanning revealed the spiracular morphology and position in detail, which are very similar to those of Argasidae 24. Although the spiracle position in Deinocroton is coincident with that of Nuttalliella , the latter has a minute spiracle with a cribose spiracular plate20. Also, the spiracle of the new family is quite different from that in Ixodidae (i.e., bigger and in a posterior position, not hidden by coxae IV 19, 25). Lastly, the ventroposterior grooves that are posterior to coxae IV and diverge towards the posterior body margin have been named herein “ posterior genital groove sections ”, despite not being connected to the longitudinal grooves that arise from the genital area. Although the origin of these posterior grooves is unclear, the set of the anterior and posterior sections is very similar in position and extension to the genital grooves of some ixodids. Other ixodids, such as Boophilus , have posterior grooves due to the presence of adanal shields; however, since Deinocroton lacks any structure resembling this shield, the posterior section of the genital groove in the new family appears to be unique among ticks.
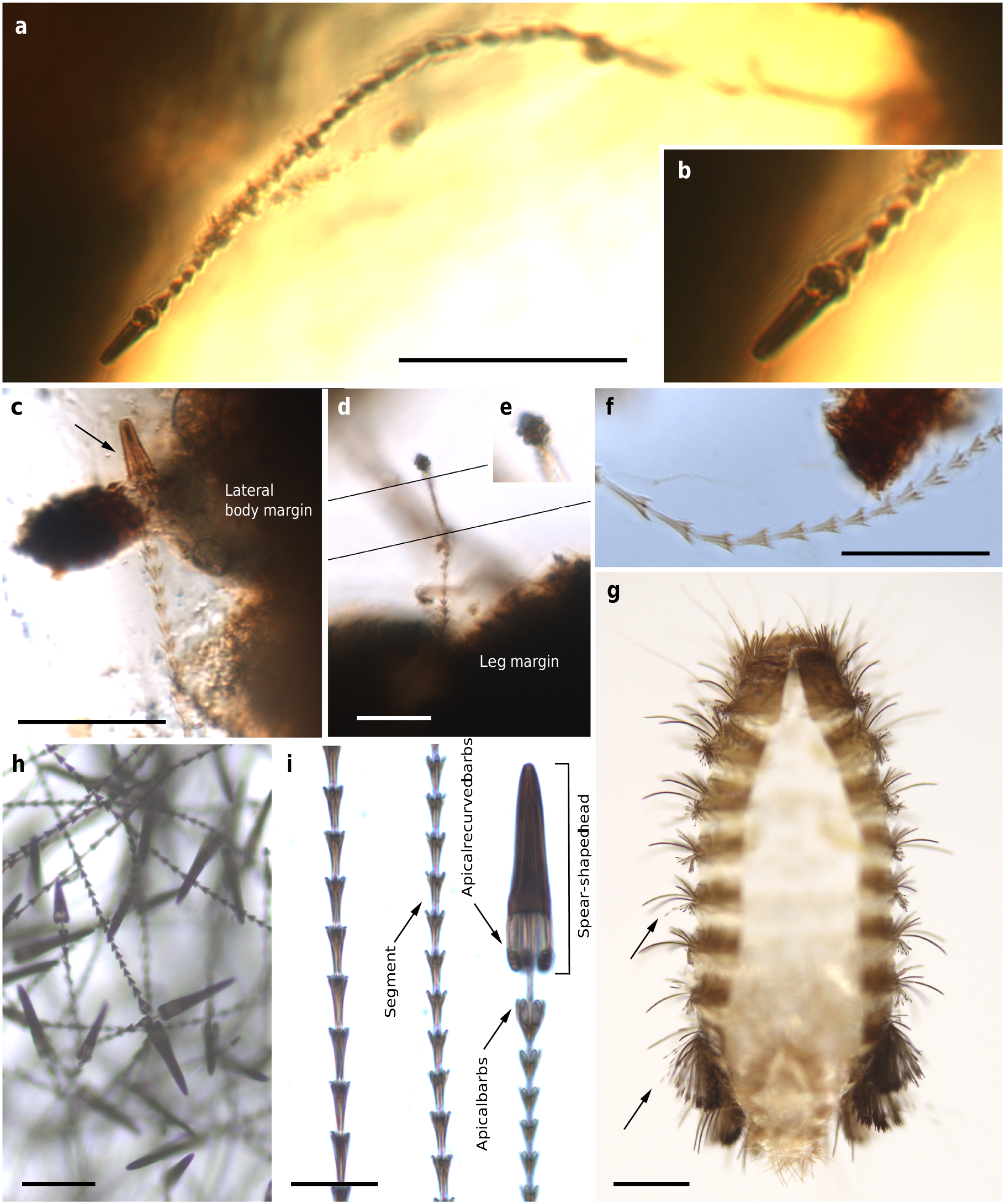
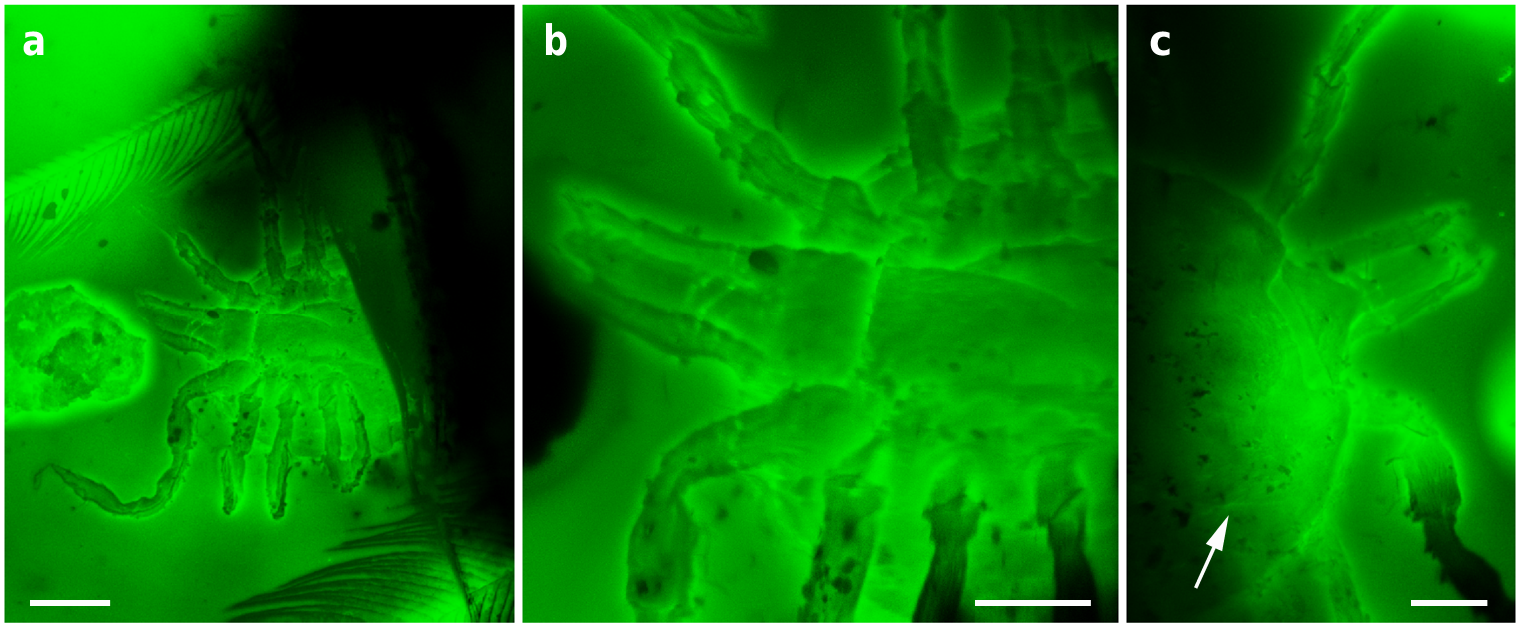
The holotype and paratype male Deinocroton , preserved together, have at least seven spear-headed, multi-segmented setae of exogenous origin attached to their bodies ( Fig. 6 View Fig. 6 ; Supplementary Fig. 4 View Fig. 4 ). The longer setae remains are 311 µm ( Fig. 6 a View Fig. 6 ; Supplementary Fig. 4 b View Fig. 4 ) and 286 µm ( Fig. 6 f View Fig. 6 ; Supplementary Fig. 4 e View Fig. 4 ) in length as preserved and contain 27 segments plus its spear-head and 23 segments, respectively. The spear-head is 27 µm long, 5 µm wide ( 11 µm in the base), more sclerotised than the rest of the seta and with six basal knobs arranged in circle. The basalmost segments are long ( 23 µm long the longest preserved) and quickly decrease in length towards the apex of the seta. The distal setal section shows short segments of similar length (ca. 9 µm long in the 20 distal segments), with the distalmost segment (that in connection with the spear-head) not differing in shape and size from the immediately preceding ones.
Despite the dilated body of the engorged specimen (paratype female), it belongs to Deinocroton draculi based on the virtually identical size and morphology of the capitulum (including the basis capituli), pseudoscutum, legs (including the relative length of leg segments), two sections of the genital groove, spiracle and anal plate. The morphoanatomical changes in the engorged specimen when compared to the three unengorged ones (attributed to engorgement) are as follows ( Figs. 3 b, c, h, m View Fig. 3 , 5 h, i View Fig. 5 , 7 View Fig. 7 ; Supplementary Table 3): ( 1) the body increased ca. 1.7 times its length, ca. 1.4 times its greatest width, and ca. 3.6 times its greatest height — this corresponds to an approximate volume change from 15.0 to 126.0 mm3 (i.e., a volume increase of ca. 8.5 times); ( 2) the dorso-ventrally planar body became inflated (more pronouncedly so medially along the longitudinal axis) and its subcircular outline became elongated (bean-shaped), particularly in the transverse medial portion of the body or area that separates the anterior and posterior sections of the genital groove; ( 3) the body integument became smooth, without evidence of the original pits; ( 4) the post-genital depressed, quadrangular area disappeared; ( 5) coxae became strongly separated from one another, particularly coxae II from III and III from IV; ( 6) the genital aperture became deformed to a plate with a globular extruded protrusion; ( 7) the spiracle was displaced to a posterior position regarding coxae IV, but without changes in its morphology and size; and ( 8) the anal plate became dilated (its greatest width increased by one-and-half times) but the anal valves remained unchanged in morphology and size. It is noteworthy that the pseudoscutum preserved its size and pits in the engorged specimen, without signs of dilation, as in the allotype. The pseudoscutum does not change its morphology with engorgement in Nuttalliella either5. The engorged Deinocroton represents the third engorged tick known in the fossil record; the other records have been found in Cretaceous Burmese amber7 and Miocene Dominican amber26.
| AMNH |
American Museum of Natural History |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |