Aegla paulensis Schmitt, 1942 s
|
publication ID |
https://doi.org/10.11646/zootaxa.4193.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:17A58F3B-AB46-4509-8CFF-A7C2A6B7E15A |
|
DOI |
https://doi.org/10.5281/zenodo.6077973 |
|
persistent identifier |
https://treatment.plazi.org/id/038A87FC-FF83-F912-FF59-FC2AFF0AB8AB |
|
treatment provided by |
Plazi |
|
scientific name |
Aegla paulensis Schmitt, 1942 s |
| status |
s |
Aegla paulensis Schmitt, 1942 s View in CoL . str.
( Figs 1 View FIGURE 1 , 10 View FIGURE 10 –11,24A–B, 25A, 26A, 27A, 28A, 29A, 30)
Aegla odebrechtii paulensis Schmitt, 1942: 490 , figure 56a–e [male holotype], pl. 27 B.
Aegla paulensis View in CoL .— Bond-Buckup & Buckup, 1994: 241, figs 49 [male holotype], 69b [distribution map].
Type material. Holotype: male [ 15.84 mm], Brazil, São Paulo, Alto da Serra do Cubatão, between Santos and São Paulo, D. Cochran coll., 26.iv.1935, coordinates and altitude not available ( USNM 80023 About USNM ) . Paratypes: 1 female [ 12.88 mm], ibidem holotype, D. Cochran coll., 25.iv.1935, coordinates and altitude not available (USNM 169112). 3 males [largest male: 16.80 mm, smallest male: 11.97 mm] and 3 females [largest female: 13.99 mm, smallest female: 12.34 mm], ibidem, D. Cochran coll., 25.iv.1935, coordinates and altitude not available (USNM 169111).
Other material examined. 3 males topotypes [largest male: 15.27 mm, smallest male: 12.20 mm], Brazil, São Paulo, Alto da Serra de Paranapiacaba Biological Reserve, 17.iv.2005, geographical coordinates, altitude and collector not available ( MZUSP 34368 View Materials ) . 16 males topotypes [largest male: 15.77 mm, smallest male: 11.05 mm], ibidem, Trilha 2– Reservatório stream, 23°46’45.6”S –046°18’37.7”W, altitude 884 m, JCB Moraes and SLS Bueno coll., 26.vi.2014 (MZUSP 34367, genetic voucher: Genbank access KU948368 View Materials ). 7 specimens [sex and size not recorded], Brazil, São Paulo, Rio Juquery (tributary of Rio Perus ), 23°20’S –046°50’W, collector and date not available (USNM 1293513). 2 juveniles [size not recorded], Brazil, São Paulo, Salesópolis, Boraceia Biological Station, collector and date not available (MZUSP 7315). 1 female and 3 juveniles [size not recorded], ibidem, Venerando stream, collector and date not available (MZUSP 7311). 2 males [size not recorded], Brazil, São Paulo, Estrada Alto da Serra—Serra do Mar, collector and date not available (MZUSP 1432). Number and sex of specimens not specified, Brazil, São Paulo, Alto da Serra, collector and date not available (MZUSP 0624). 1 male and 2 females [size not recorded], Brazil, São Paulo, Jaraguá State Park, collector and date not available (MZUSP 7317). 3 males, 1 female and 1 juvenile [size not recorded], ibidem, collector and date not available (MZUSP 7410). 4 males and 3 females [size not recorded], Brazil, São Paulo, Perus, collector and date not available (MZUSP 0703). 4 males [size not recorded], Brazil, São Paulo, Embu Guaçu, collector and date not available (MZUSP 7310).
Type-locality. Alto da Serra do Cubatão /Paranapiacaba, between cities of Santos and São Paulo, São Paulo, Brazil.
Geographical distribution. Known only from the type-locality.
Diagnosis. Rostrum triangular, base narrow, curved downward, extending beyond distal apex of compound eyes. Subrostral process on proximal half, well developed, high, acute triangular, oriented downward. Orbital spines well developed. Epibranchial area with one corneous scale near anterolateral angle. Areola subrectangular. Anteromesial region of third thoracic sternite tapered. Chelipeds large; palmar crest rectangular with margin lobulate. Uropods wide. Posterolateral margin of telson slightly concave mesially.
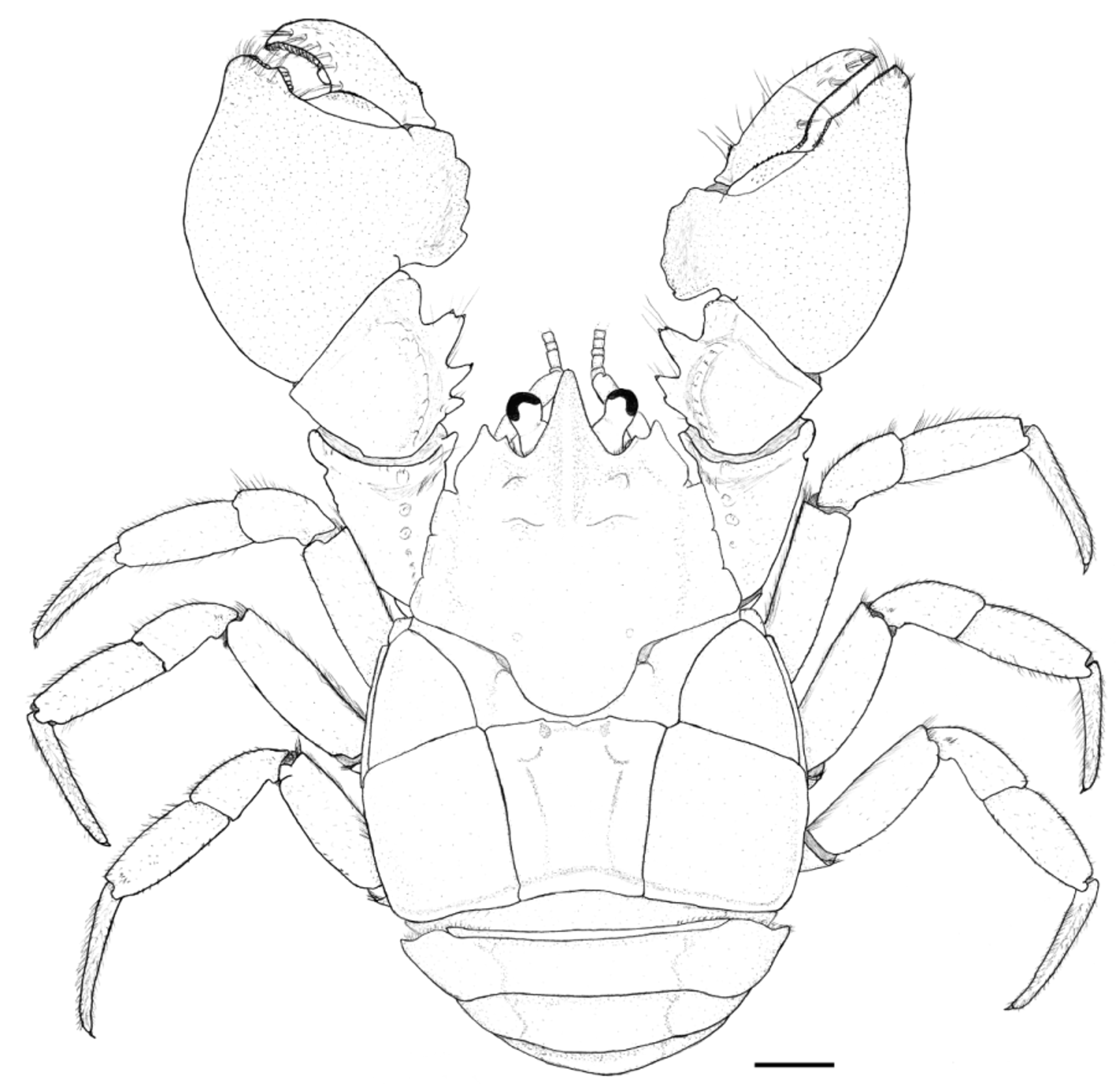
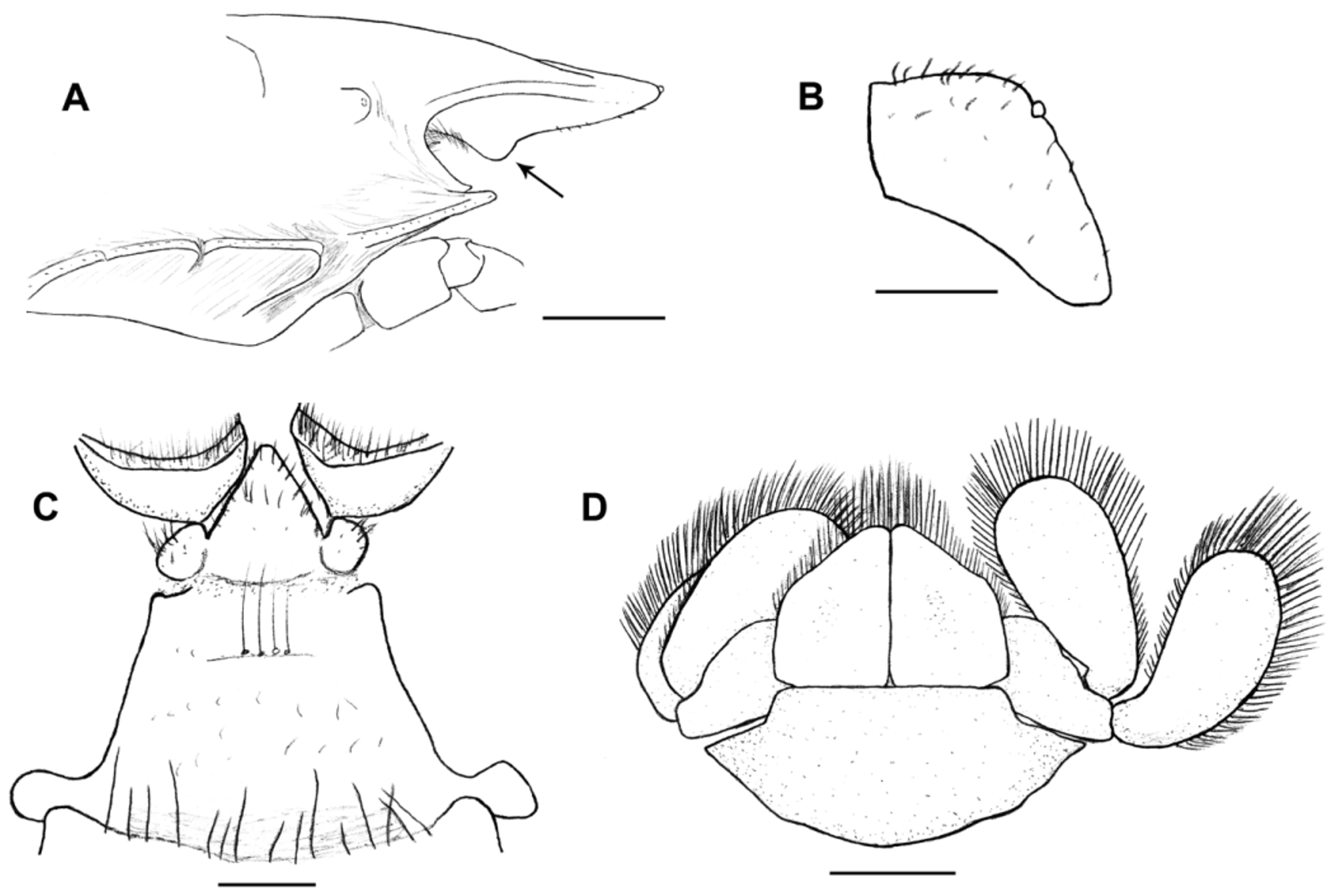
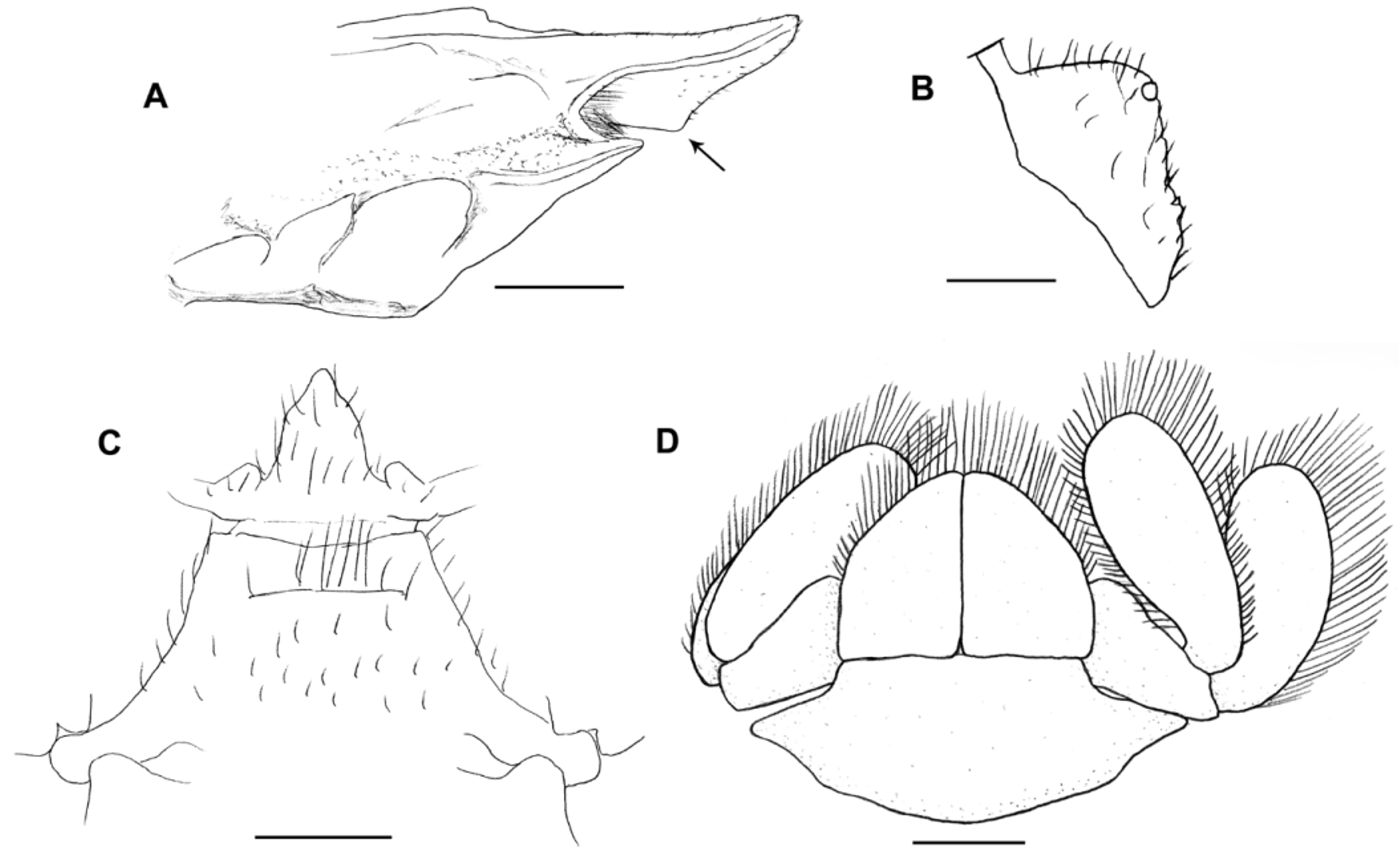
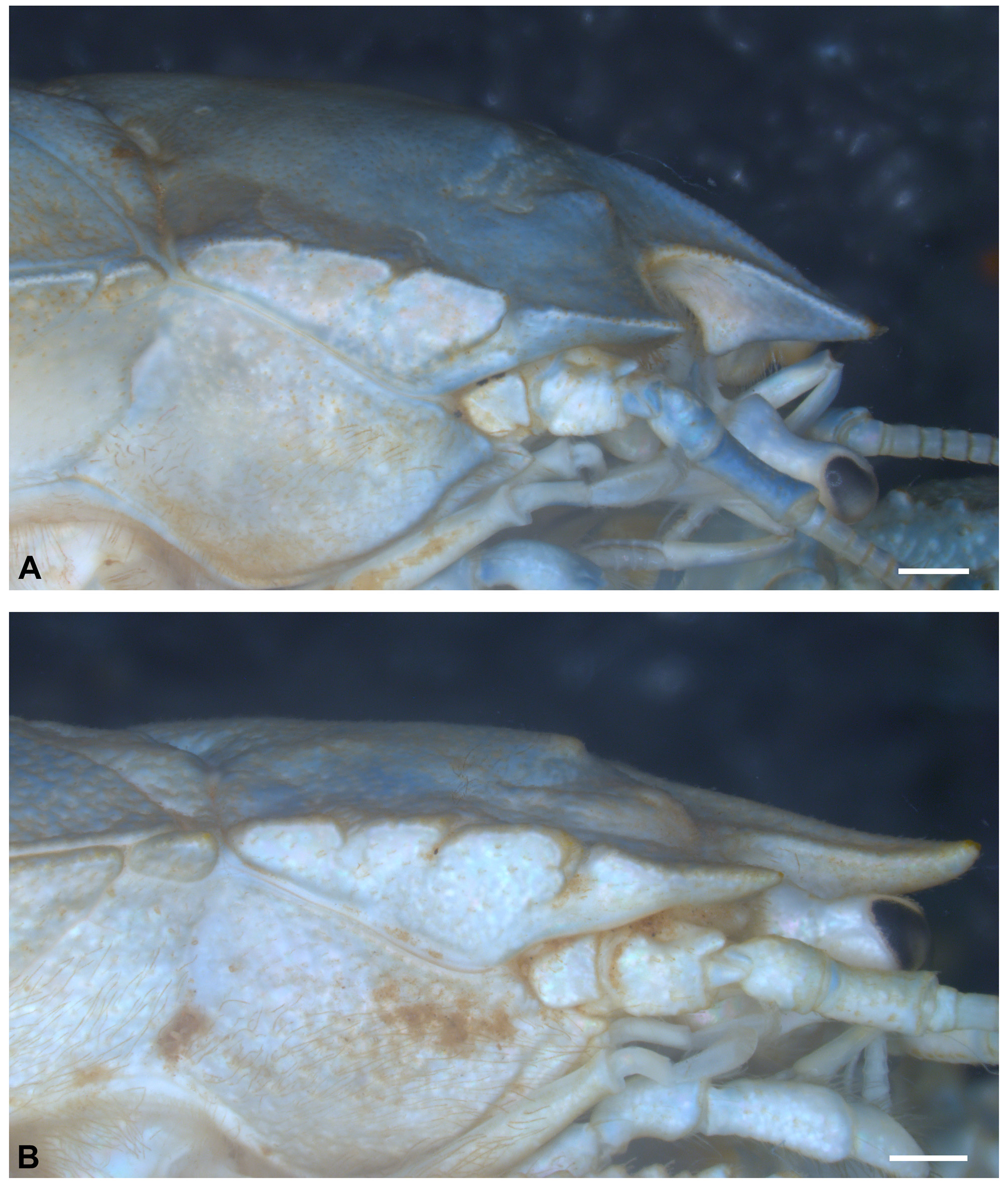
Description of the male holotype. Carapace moderately convex, gastric region swollen, dorsal surface scabrous, covered with punctations with small setae. Rostrum triangular, base narrow (value = 0.90), curved downward, extending beyond distal apex of compound eyes, with small corneous scales on lateral margins and tip ( Fig. 10 View FIGURE 10 ). Rostral carina beginning at level of protogastric lobes, extending to near apex, with small corneous scales. Subrostral process well developed, on proximal half of subrostral margin, tip rounded, anterior and posterior margins forming angle of about 86° ( Fig. 11 View FIGURE 11 A).
Eyestalk and cornea well developed. Orbital sinus U-shaped, with plumose setae subventrally. Orbital spines well developed, rounded apically, with terminal small corneous scale. Anterolateral spines rounded apically with terminal small corneous scale, straight, not reaching basal margin of cornea ( Fig. 10 View FIGURE 10 ).
Epigastric prominences and protogastric lobes pronounced, with small corneous scales.
Gastric area prominently inflated in relation to hepatic lobe and rostrum in lateral view ( Fig. 10 View FIGURE 10 ). Gastric pits small, smooth.
Demarcation between hepatic lobes well defined. Lateral margins of hepatic lobes with small corneous scales and small setae ( Fig. 10 View FIGURE 10 ).
Transverse dorsal linea (TDL) slightly sinuous throughout its extension, sinuosity more pronounced mesially. Areola subrectangular (value = 2.25). Cardiac area trapezoidal (value = 1.51) ( Fig. 10 View FIGURE 10 ).
Epibranchial area slightly elongated, triangular shaped, anterolateral angle blunt with small corneous scale, margins with scattered small simple setae ( Fig. 11 View FIGURE 11 B).
Anteromesial region of third thoracic sternite tapered with scattered, simple setae over the surface. Fourth thoracic sternite with anterolateral angles moderately produced ( Fig. 11 View FIGURE 11 C).
Chelipeds unequal in size ( Fig. 10 View FIGURE 10 ). Major cheliped. Dactylus: dorsal margin and outer surface granulate; inner surface smooth; proximal lobe on dorsal margin blunt; cutting margin with lobular basal tooth well developed proximally, with flattened corneous scales, followed by row of wide corneous scales up to distal end; row of tufts of long, simple setae next to cutting margin. Propodus: outer surface granulate; palm high (value = 3.32); palmar crest rectangular, margin lobulate, outer surface excavated; cutting margin of fixed finger with lobular basal tooth well developed proximally, with flattened corneous scales, followed by row of wide corneous scales up to distal end; scattered tufts of long simple setae over inner surface and alongside inner and outer surfaces next to cutting margin. Carpus: dorsal margin with three spines of unequal size; proximal spine smallest, naked, partially merged with small tubercle at base; mesial spine with small terminal corneous scale; distal spine longest, double-tipped, each tip with small corneous scale; subterminal lobe of moderate size, blunt, with small corneous scales apically; inner surface with group of three tubercle of unequal size (the two largest ones with one small corneous scale each) near dorsal margin and long setae; outer surface with carpal ridge high, formed by tubercles. Merus: dorsal margin with one tubercle; dorsolateral edge with two larger tubercles distally, followed by row of tubercles decreasing in size proximally; ventromesial edge with three spines decreasing in size proximally, tipped by corneous scale and one proximal, naked tubercle; ventrolateral border with row of several small tubercles increasing slightly in size distally, penultimate distal tubercle with terminal corneous scale. Ischium: dorsolateral edge with distal, naked spine; ventromesial border with distal tubercle with terminal corneous scale; two proximal tubercles (proximalmost naked); ventrolateral border smooth.
Minor cheliped similar to major cheliped except as noted hereafter. Dactylus: cutting margin with lobular basal tooth weakly developed, followed by row of narrow corneous scales up to distal end. Propodus: palmar crest disciform, margin weakly lobulated; cutting margin of fixed finger with lobular basal tooth weakly developed formed by narrow corneous scales, followed by row of narrow corneous scales up to distal end. Carpus: two spines of unequal size on dorsal margin; distalmost spine tipped by two spiny, corneous scales; proximalmost spine partially merged with naked tubercle at base, tipped by one spiny, corneous scale; one additional small naked tubercle proximally; inner surface with one tubercle well developed with spiny corneous scales and long setae terminally. Merus: ventromesial edge with five tubercles increasing slightly in size distally, proximalmost naked, next four tipped by corneous scales; ventrolateral border with row of several small, naked tubercles, the two distalmost are larger. Ischium: dorsolateral edge with one naked tubercle; ventromesial border with one large tubercle with terminal corneous scale, followed by three smaller tubercles proximally (proximal two naked); ventrolateral border smooth.
Second, third and fourth pereiopods morphologically similar, except where noted. Dactyli with several rows of setal tufts on general surface. Propodi, meri and ischii with scattered, short, long setae concentrated mainly along dorsal margin. Carpi with scattered, short, long setae concentrated mainly along dorsal margin; second and third pereiopods with one small corneous scale each on distal portion of dorsal margin ( Fig. 10 View FIGURE 10 ).
Fifth pereiopod reduced, chelate. Dactylus small, flattened, forming setose minute chela with propodus. Sexual tube long, narrow, opening on coxa ( Figs. 24A–B View FIGURE 24. A – L ).
Pleopods 2 and 5 absent, and 3 and 4 present as buds.
Anterolateral angle of second abdominal epimeron and ventral angles of third and fourth abdominal epimera well defined, unarmed.
Uropods well developed, wide ( Fig. 11 View FIGURE 11 D).
Telson with anterolateral and posterolateral margins well-differentiated; posterolateral margin slightly concave mesially ( Fig. 11 View FIGURE 11 D).
Variations. In comparison to the holotype the inflation of the gastric area and the downward curvature of rostrum is less pronounced in the paratypes, and the shape of the subrostral process in the paratypes and topotypes are somewhat more pronounced and acute ( Fig. 26A View FIGURE 26. A – B ). In the paratypes and topotypes the palmar crest of both minor and major chelipeds may appear slightly disciform and weakly excavate on outer surface. In some topotypes (MZUSP 34367 and 34368) the rostral carina may originate more anteriorly in relation to the protogastric lobes; the small corneous scale on the epibranchial area may be absent; the cutting margin of the dactylus and propodus of major chela may have narrow corneous scales and the carpal ridge may have low tubercles.
Remarks. Aegla paulensis was established by Schmitt (1942) as a subspecies of A. odebrechtii Müller, 1876 . Bond-Buckup & Buckup (1994) have shown in detail that A. paulensis Schmitt differs in many ways from A. odebrechtii Müller and, therefore, deserves full specific status. While A. odebrechtii Müller is currently restricted to south Brazil ( Santa Catarina and north of Rio Grande do Sul), A. paulensis has been traditionally regarded as displaying much larger geographical distributions that include three currently isolated river basins: the Ribeira de Iguape, the Tietê, and the Paraíba do Sul. However, based on morphological and molecular evidences A. paulensis is regarded herein as a complex of seven species, which includes A. paulensis Schmitt, 1942 s . str., A. rosanae Campos Jr., 1998 n . stat., removed from the synonymy of A. paulensis s. str., A. lancinhas Bond-Buckup & Buckup in Santos et al., 2015 , A. vanini n. sp., A. japi n. sp., A. jaragua n. sp. and A. jundiai n. sp.. Aegla paulensis s. str. is unique in the species complex in having wide uropods ( Fig. 11 View FIGURE 11 D), whereas all other species mentioned above have narrow uropods ( Figs. 13 View FIGURE 13 D, 15F, 17D, 19D, 21D, 23D). Aegla paulensis s. str. is further characterized by a unique combination of characters including: (i) rostrum distinctly curved downward ( Fig. 26A View FIGURE 26. A – B ); (ii) rostrum long ( Figs. 10 View FIGURE 10 , 25A View FIGURE 25. A – F ); (iii) orbital spines well developed ( Fig. 10 View FIGURE 10 ); (iv) areola subrectangular ( Figs. 10 View FIGURE 10 , 25A View FIGURE 25. A – F ); (v) margin of palmar crest of both chelipeds lobulate ( Fig. 10 View FIGURE 10 ); (vi) anteromesial margin of the third thoracic sternite tapered distally ( Figs. 11 View FIGURE 11 C, 29A); and (vii) posterolateral margin of the telson slightly concave mesially ( Fig. 11 View FIGURE 11 D). The morphological differences between the members of this species complex are discussed below.
The specimens from São Paulo (Boraceia, Salesópolis, MZUSP 7315 View Materials ; Venerando stream, Salesópolis, MZUSP 7311 View Materials ; Perus, MZUSP 0703 View Materials ; Embu-Guaçu, MZUSP 7310 View Materials ; Jaraguá , MZUSP 7317 View Materials and MZUSP 7410 View Materials ) assigned to Aegla paulensis by Bond-Buckup & Buckup (1994: 242) actually do not belong to Aegla paulensis s. str. and are therefore reassigned to other species (see below).
Examination of specimens from Perus (Juquery River, state of São Paulo, USNM 1293513), previously identified as Aegla odebrechtii paulensis , revealed that this material differs from Aegla paulensis s. str. by the following combination of characters: (i) apex of rostrum not reaching distal apex of compound eyes (whereas in A. paulensis the rostrum extends beyond the distal apex of the eyes); (ii) elongation of the epibranchial area distinctly pronounced (whereas in A. paulensis the epibranchial area is only slightly elongated); (iii) anteromesial margin of third thoracic sternite truncate distally (whereas in A. paulensis it tapers distally); (iv) proximal lobe on dorsal margin of dactylus of chelipeds with narrow high tubercle (whereas in A. paulensis that lobe is wide and blunt). Further investigation will be required to assess the taxonomic identity of those USNM specimens from Perus.
Biology. The reproductive period, fecundity, population structure (sex ratio and temporal variation in size-class distribution), habitat preference of newly-hatched juveniles and migration of adults have been studied by López (1965).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Aegla paulensis Schmitt, 1942 s
| Moraes, Juliana Cristina Bertacini, Terossi, Mariana, Buranelli, Raquel Corrêa, Mantelatto, Fernando L. & Bueno, Sérgio Luiz De Siqueira 2016 |
Aegla paulensis
| Bond-Buckup 1994: 241 |
Aegla odebrechtii paulensis
| Schmitt 1942: 490 |