Urticaleoxylon, 2023
|
publication ID |
https://doi.org/10.5070/P9401462457 |
|
DOI |
https://doi.org/10.5281/zenodo.13890964 |
|
persistent identifier |
https://treatment.plazi.org/id/038AF505-A324-963F-57C0-FE34FE4E9B92 |
|
treatment provided by |
Felipe |
|
scientific name |
Urticaleoxylon |
| status |
sp. nov. |
URTICALEOXYLON STEVENSII SP. NOV.
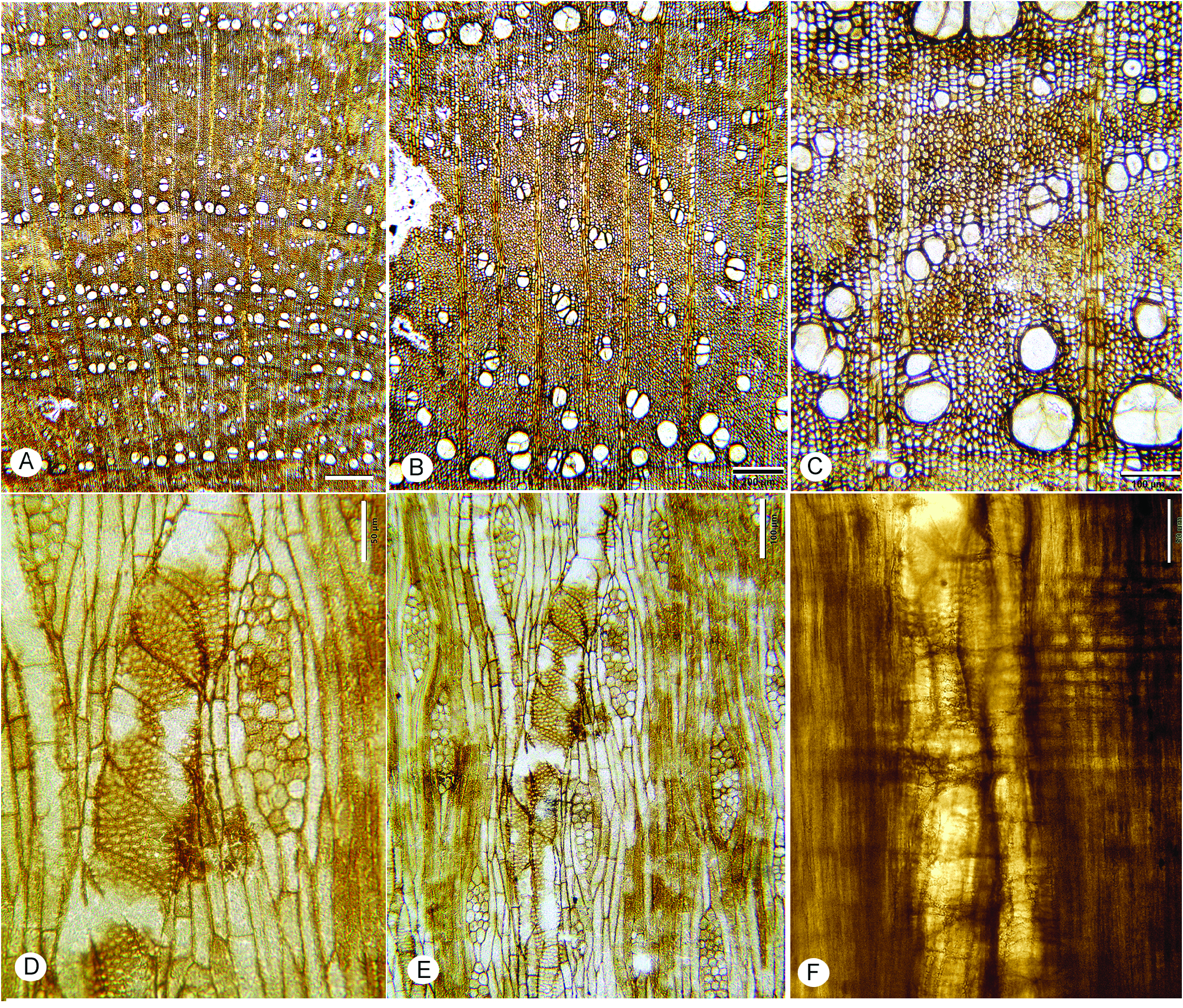
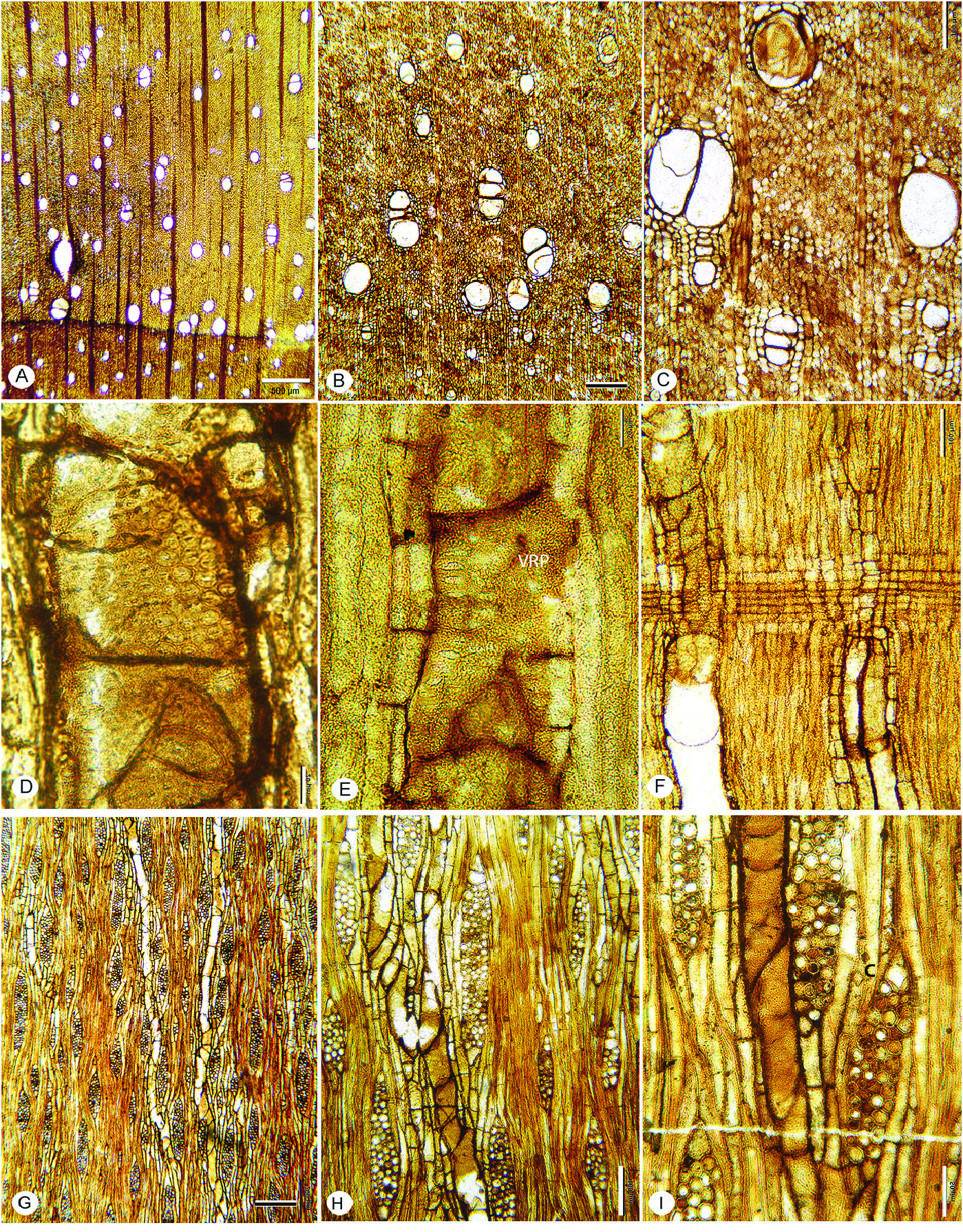
FIG. 13A–I View Figure 13
Diagnosis— Same as genus.
Holotype — UF 278-84893 , estimated maximum diameter 8 cm.
Occurrence— Dietz Hill ( UF 278).
Etymology— Named for Peter F. Stevens in thanks for the creation and maintenance of Angiosperm Phylogeny Website.
Description— Growth rings present, marked by radially narrow fibers, and marginal axial parenchyma ( Fig. 13A–C View Figure 13 ). Wood semi-ring-porous; vessels solitary and in radial multiples of 2–3 (rarely 4) ( Fig. 13A View Figure 13 ); mean tangential diameter of earlywood vessels 119 µm (SD=18), range 78–150 µm. Perforation plates simple ( Fig. 13E, F, H, I View Figure 13 ); intervessel pits alternate ( Fig. 13D View Figure 13 ), horizontal diameter 8–11 µm. Vessel-ray parenchyma pits horizontally elongate, simple or with reduced borders ( Fig. 13E View Figure 13 ). Mean vessel element length 377 (SD=148) µm; range 153–594 µm vessel element end walls inclined to nearly horizontal. Thin-walled tyloses ( Fig. 13D, I View Figure 13 ).
Fibers thin- to thick-walled, mostly non-septate, occasionally septate, pitting not observed ( Fig. 13H, I View Figure 13 ).
Axial parenchyma marginal, scanty paratracheal, vasicentric, more abundant in the latest latewood with some aliform-confluent ( Fig. 13B, C View Figure 13 ); strands of 2–4 cells ( Fig. 13H, I View Figure 13 ).
Rays 1–6 seriate, mostly 4–5-seriate; uniseriate rays short, usually less than five cells high ( Fig. 12G–I View Figure 12 ); average multiseriate ray height 448 (SD=199) µm, 216–878 µm. Rays homocellular, composed of procumbent cells, and heterocellular with 1–2 marginal rows of square/ upright cells, rarely more; 4–6 per mm.
Occasional solitary prismatic crystals in upright marginal ray cells ( Fig. 13H, I View Figure 13 ).
Storied structure and oil/mucilage cells not observed.
Comments— Fine fungal hyphae are common in the sample and can be confused with septate fibers. Most fibers appear non-septate, but there are a few septate fibers as well.
Comparisons with extant plants— We searched InsideWood multiple times using slightly different combinations of features. In all searches we used: presence of distinct growth ring boundaries (1p), wood semi-ring-porous (4p), absence of exclusively solitary vessels, distinct vessel arrangement, and radial multiples of 10 or more common (6a, 7a, 8a, 9a, 10a), presence of simple perforation plates and alternate intervessel pits that are not minute (13p, 22p, 24a), vessel-ray parenchyma pits with reduced borders and horizontally elongate (32p), non-septate fibers with simple pits (61p, 66p), vasicentric axial parenchyma (79p), maximum ray width 4-10-seriate (98p), rays not composed of all upright cells or markedly heterocellular or with intermixed procumbent and square/upright cells (105a, 108a 109a), storied structure and oil/mucilage cells absent (118a, 120a, 124a 125a 126a), radial canals absent (130a), prismatic crystals in square/upright ray cells (136p, 137p).
Figure 13. Caption on pg. 28.
A search using the features listed above and not allowing mismatches returned only Cannabaceae ( Celtis ) and Moraceae ( Morus L., 1753; Maclura Nutt., 1818 ). Semi-ring-porous Celtis commonly have vessel clusters and the diffuse-porous species have more axial parenchyma. Axial parenchyma is much more abundant and there are more vessel multiples in Maclura (ter Welle et al., 1986) . A search allowing a single mismatch returned Antiaris Lesch. (1810) and Helicostylis Trécul (1847) , both Moraceae . When we added presence of both septate and non-septate fibers (65p 66p), there were no matches, but allowing one mismatch also returned Cannabaceae ( Celtis ) and Moraceae ( Antiaris , Morus , and Maclura ) as well as a Toxicodendron Mill. (1754; Anacardiaceae R. Brown, 1818a ) differing in ray size and appearance of vessel-ray parenchyma pits; Vitex L. (1753) spp. ( Verbenaceae J. Saint-Hilaire, 1805 ), which only has septate fibers; Cedrela P. Browne (1756) ( Meliaceae Jussieu, 1789 )), which can be excluded because of its small intervessel pits and vessel-ray parenchyma pits being similar to the intervessel pits.
Porosity in present-day Celtis and Morus woods varies, with temperate zone species being ring-porous, subtropical species semi-ring-porous to diffuse-porous, and tropical species diffuse-porous. There is also variation within species. The FFPRI’s Database of Japanese Woods (https://db.ffpri.go.jp/WoodDB/JWDB-E/home.php) has images of multiple samples of Morus alba L. (1753), M. boninensis Koidz. (1917) and Broussonetia papyrifera (L.) L’Hér.ex Vent.(1799). Most are ring-porous with latewood vessels in clusters tending to be arranged in wavy tangential bands, but some samples have porosity, vessel grouping and arrangement similar to UF 278-84893: Mo. alba (TWTw 17381); B. papyrifera (TWTw 19291, TWTw 23325). One of the two FFPRI samples of Mo. bonienesis (TWTw 10933) also is similar.
Kew’s Plants of the World accepts 19 species of Morus ( POWO 2023) ; ter Welle et al (1986) described the wood anatomy of seven species; InsideWood has descriptions and/or images of eleven species.
We infer that this Dietz Hill (UF 278) wood belongs to the group of families once placed in the Urticales , but now considered to belong to the Rosales and informally referred to as the urticalean rosids.
Consequently, we propose the genus Urticaleoxylon to accommodate such woods.
Comparisons with fossil plants— There are no fossil woods in the InsideWood database that exactly match the criteria used to search the modern wood database.
Celtis popsii Wheeler and Manchester (2021) occurs at the Post Hammer location (UF 279). It differs from this Dietz Hill (UF 278) wood because its latewood vessels are in clusters arranged in wavy tangential bands, rays are of two size classes, and sheath cells are present.
At the older middle Eocene Nut Beds locality, there were three woods placed in the Urticales ( Wheeler and Manchester 2002). Scottoxylon eocenicum Wheeler and Manchester (2002) was one of the most common Clarno Nut Beds woods with more that 50 samples. It differs from this Dietz Hill (UF 278) woods as axial parenchyma is more common, rays are larger, and none of the samples had crystals. Doweld (2021) proposed that Scottoxylon be replaced because it was a homonym of an earlier genus name, Scotoxylon Vogellehner (1968) ; unfortunately, the name he proposed, Alloceltidoxylon Doweld (2021) , implies affinities with Celtis . We retain Scottoxylon because it was validly published (note this spelling differs from Scotoxylon Vogellehner ; the botanical code of nomenclature recommends against, but does not prohibit, similar spelling. Clarno Urticalean Wood Types I and II also had more abundant axial parenchyma than is known for Celtis .
| UF |
Florida Museum of Natural History- Zoology, Paleontology and Paleobotany |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |