Diorhabda sublineata ( Lucas, 1849 ) REVISED STATUS
|
publication ID |
https://doi.org/ 10.11646/zootaxa.2101.1.1 |
|
persistent identifier |
https://treatment.plazi.org/id/038C2C5B-AC36-FFE8-3B91-FD8BF235D03A |
|
treatment provided by |
Felipe |
|
scientific name |
Diorhabda sublineata ( Lucas, 1849 ) REVISED STATUS |
| status |
|
Diorhabda sublineata ( Lucas, 1849) REVISED STATUS subtropical tamarisk beetle
( Figs. 5, 6 View FIGURES 1–9 , 16 View FIGURES 14–18 , 21 View FIGURES 19–23 , 26 View FIGURES 24–28 , 31 View FIGURES 29–33 , 36 View FIGURES 34–38 , 41 View FIGURES 39–43 , 45 View FIGURES 44–47 )
Galeruca sublineata Lucas, 1849:542 (Type locality: Hippône [Annaba], Algeria; as Galleruca ).
Galeruca elongata: Reiche and Saulcy, 1858:42 (part; France, Egypt, Algeria; as Galleruca ); Joannis, 1866:83 (part, monograph, France, as Galleruca ).
Diorhabda elongata: Heyden et al., 1891:375 (part, catalog for Europe and Caucasus, southern Europe), Bedel, 1892:158 (part, France, as Dirrhabda); Weise, 1893:635 (part, Algeria), 1925:225 ( Egypt); Corréa de Barros, 1924:9 (part, Portugal); Peyerimhoff, 1926:359 (part, Algeria, Senegal, hosts); Laboissière, 1934:54 (part, France, Senegal, host); Ogloblin, 1936:79 (part; European and African coast of Mediterranean Sea View in CoL , south to Senegal); Normand, 1937:126 ( Tunisia); Kocher, 1958:109 ( Morocco, to 2,000 m); Hopkins and Carruth, 1954:1129 (part, Spain, host); Jolivet, 1967:331 (part, Morocco, Mediterranean, hosts); Torres Sala, 1962:327 (part, Comunidad Valenciana, Spain); Petitpierre, 1988:93 (part, Spain); Gruev and Tomov, 1998:70 (part, Mediterranean); Kovalev, 1995:78 (part, southwest Palaearctic); Campobasso et al., 1999:145 (part, host, Europe and Middle East); Anonymous, 2001:52 N (part, Africa); Chatenet, 2002:223 (part, France, Spain, Morocco, Algeria, Tunisia); DeLoach et al., 2003a:230 (part, Tunisia [Sfax]), 2003b:126 (part, host range, North Africa, France), (2008, in prep.) (part, Sfax, Tunisia); Milbrath et al., 2003:225 (part, Sfax, Tunisia); Riley et al., 2003:69,189 (part, catalog of North America [introduced]); Warchalowski, 2003:328 (part, taxonomic keys, Mediterranean Region); DeLoach and Carruthers, 2004a:13, 2004b:311 (part, Tunisia [Sfax]); Lopatin et al., 2004:127 (part, Mediterranean); Dudley, 2005a:13, 2005b:42 N (part, biological control, ex: Tunisia); Milbrath and DeLoach, 2006a:32, 2006b:1379 (part, host specificity, Sfax, Tunisia); Dudley et al., 2006:137 (part, host range, Sfax, Tunisia); Milbrath et al. (2007). (part, biology, Sfax, Tunisia); Bean and Keller (in prep.) (part, diapause induction, Sfax, Tunisia); DeLoach (2008); Moran et al. in press (host range of D. sublineata [ex: Sfax, Tunisia] × D. elongata [ex: Crete] hybrid); Thompson et al. (in prep.) (part, laboratory hybridization, Sfax, Tunisia).
Diorhabda elongata var. sublineata: Weise, 1893:1132 (part, Algeria).
Diorhabda sublineata: Boehm, 1908:68 ( Egypt, as Dirrhabda).
Diorhabda elongata ab. sublineata: Weise, 1924:78 (part, world catalog, Algeria); Winkler, 1924 –1932:1307 (part, Palearctic catalog, Algeria); Laboissière, 1934:53 (part, France, Algeria); Normand, 1937:126 (part, Tunisia); Alfieri, 1976:233 ( Egypt, on Tamarix spp. ); Warchalowski, 2003:328 (part, taxonomic keys, Mediterranean Region).
Diorhabda elongata ab. carinata: Laboissière, 1934:54 (part; Narbonne, France).
Diorhabda elongata ab. bipustulata Normand, 1937:126 (Type locality: Kairouan, Tunisia) (NEW SYNONYM); Wilcox, 1971:63 (world catalog).
Diorhabda elongata sublineata: Gressitt and Kimoto, 1963a:407 (part; North Africa, West Asia; not Asia Minor, China, and Mongolia); Wilcox, 1971:63 (part, world catalog, North Africa, West Asia); DeLoach et al., 2003b:126 (part, host range, North Africa).
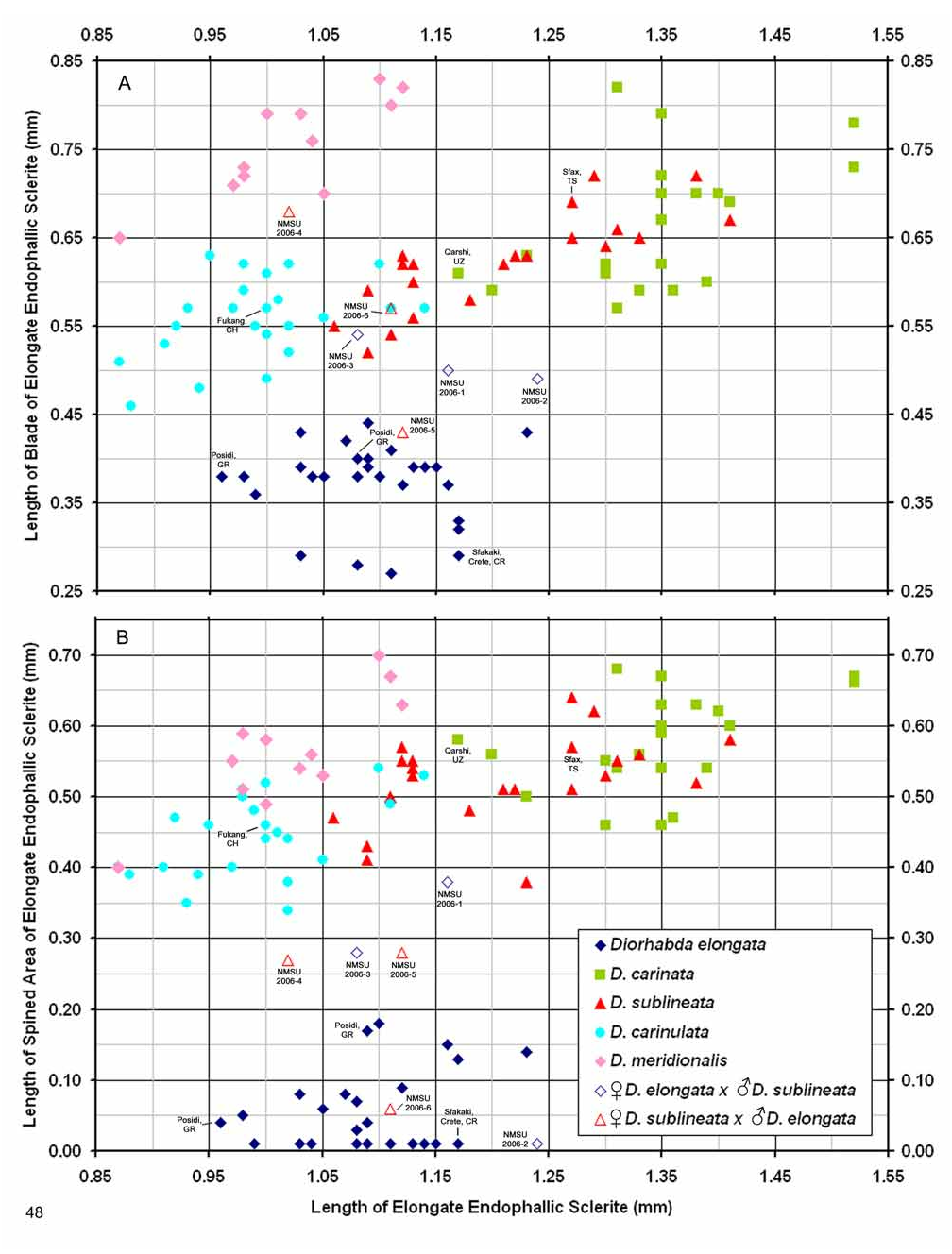
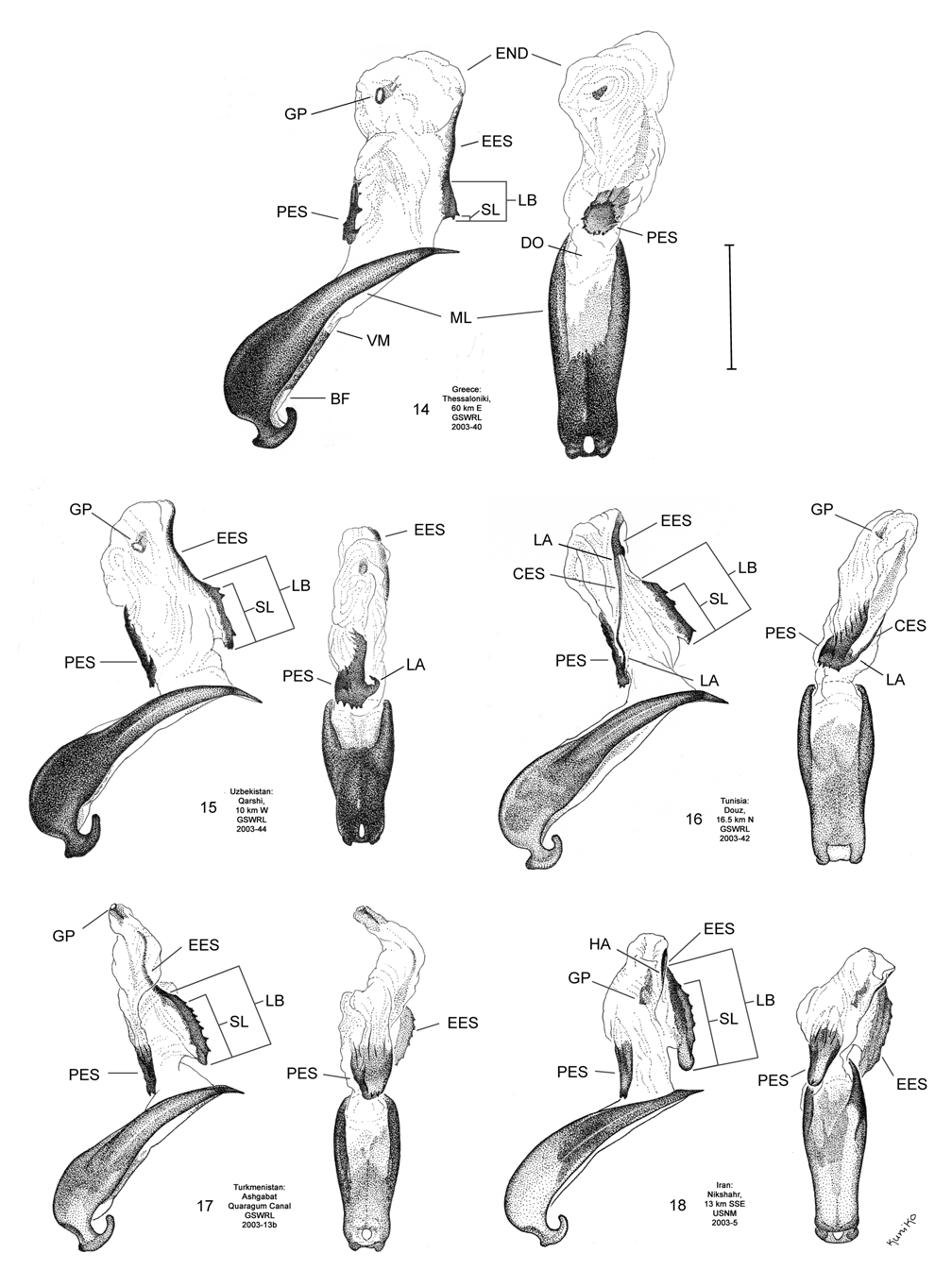
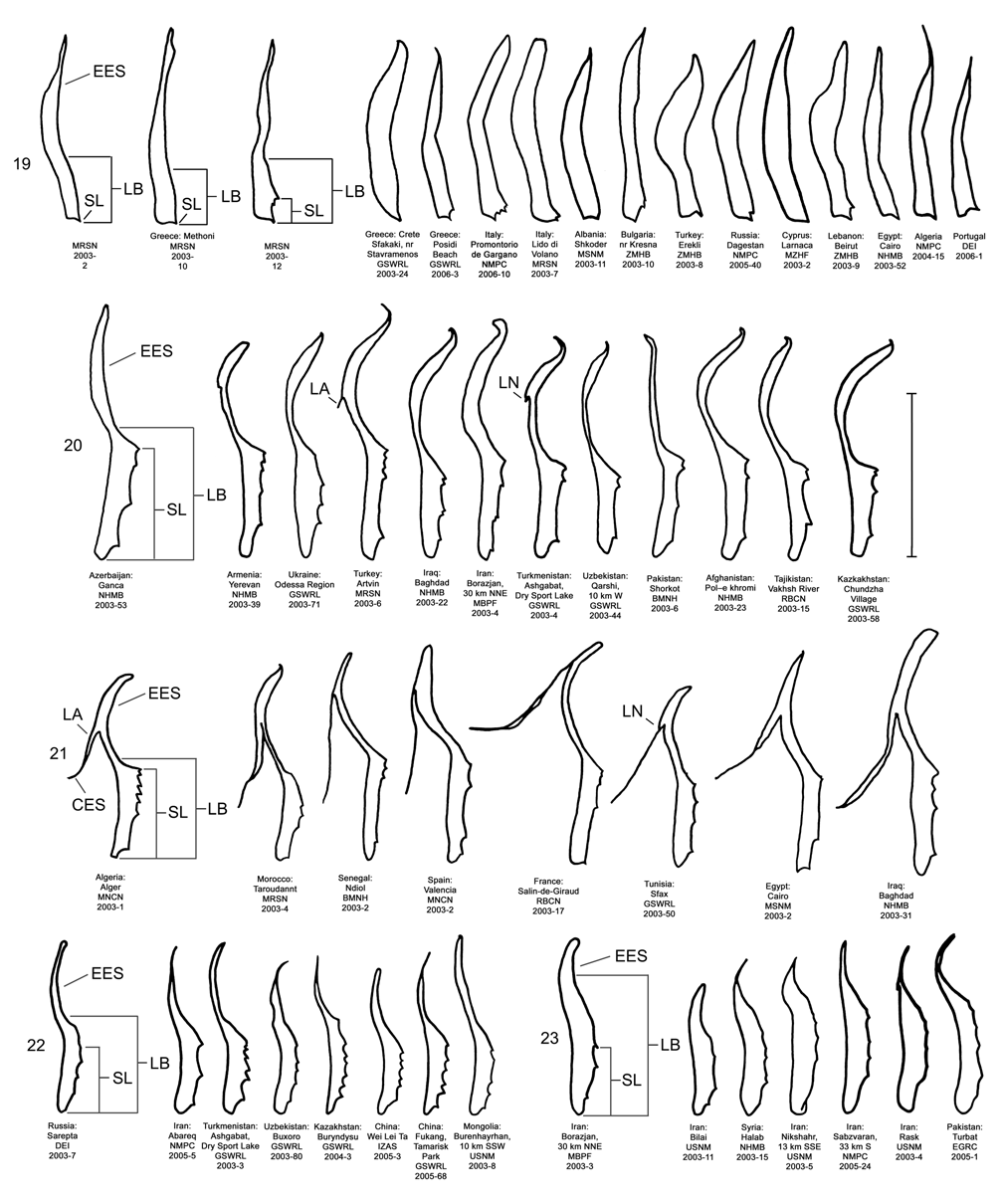
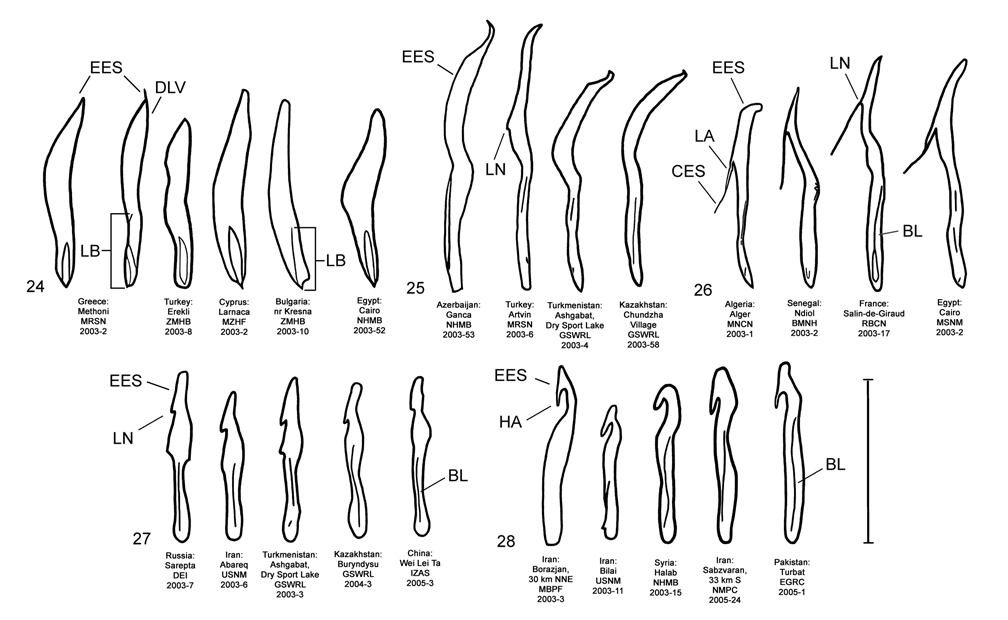
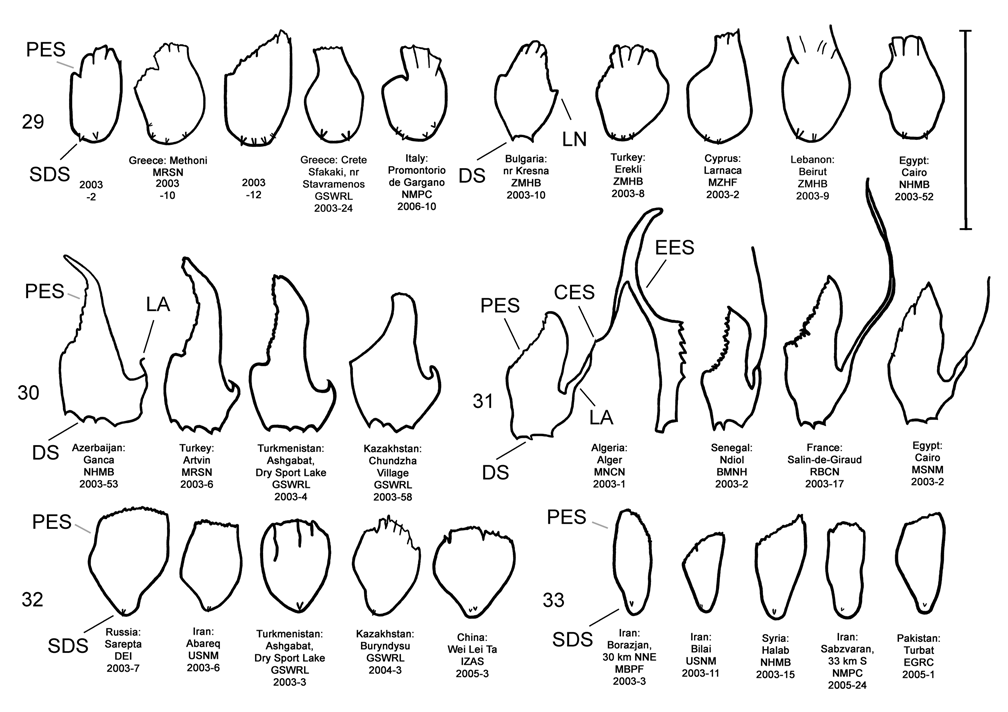
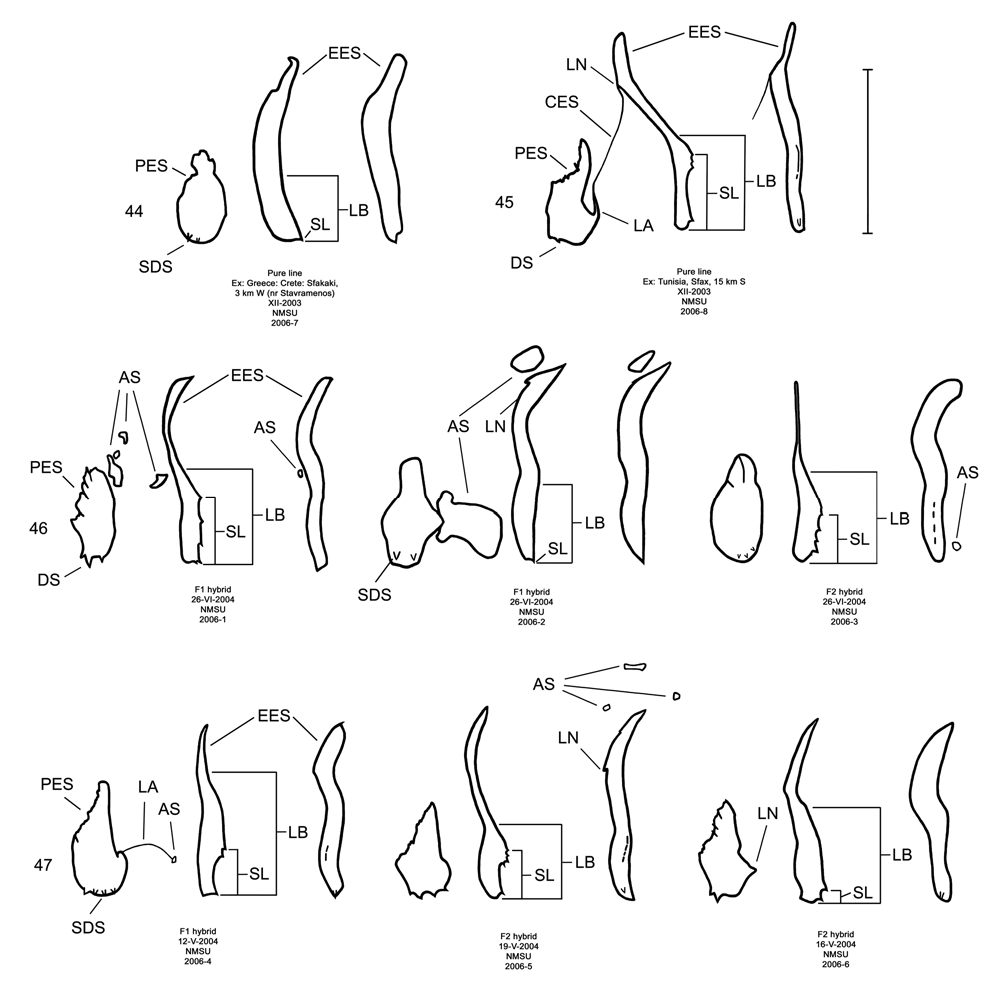
Male. Genitalia. Male D. sublineata may be distinguished from all other species in the D. elongata group by the unique presence of a connecting endophallic sclerite (CES) from the palmate endophallic sclerite (PES) to the elongate endophallic sclerites (EES) ( Figs. 16 View FIGURES 14–18 , 21 View FIGURES 19–23 , 26 View FIGURES 24–28 , 31 View FIGURES 29–33 ). In weakly sclerotized teneral adult D. sublineata , the CES can appear to evanesce and be faint. Diorhabda elongata , D. carinata , D. carinulata and D. meridionalis all lack the CES ( Figs. 14–15, 17–20, 22–25, 27–30, 32–33 View FIGURES 14–18 View FIGURES 19–23 View FIGURES 24–28 View FIGURES 29–33 ), but darkly sclerotized D. carinata may uncommonly bear a faint lateral line in place of a CES. Additional characters of the EES and PES, similar to those found in D. carinata , can be used to separate D. sublineata from D. elongata , D. carinulata , and D. meridionalis . The PES of D. sublineata ( Figs. 16 View FIGURES 14–18 , 31 View FIGURES 29–33 ) always bears a strong lateral appendage and the distal margin is truncate-serrate with two to six (commonly three to four) usually distal spines, a maximum of one spine being subdistal. In contrast, the PES of D. carinulata ( Figs. 17 View FIGURES 14–18 , 32 View FIGURES 29–33 ) and D. meridionalis ( Figs. 18 View FIGURES 14–18 , 33 View FIGURES 29–33 ) lacks a lateral appendage (but it may bear a lateral notch) and the distal margins of the PES are narrowly rounded and generally smooth with one or two small subdistal spines that sometimes project beyond the distal margin. The PES of D. elongata also lacks a lateral appendage and is usually rounded with one to five usually subdistal spines with a maximum of two of these spines being distal ( Figs. 14 View FIGURES 14–18 , 29 View FIGURES 29–33 ). The elongate endophallic sclerite in D. sublineata always bears a basally pointed lateral appendage (LA) or lateral notch (LN) serving as a point of attachment for the CES ( Figs. 16 View FIGURES 14–18 , 21 View FIGURES 19–23 ), but these are always lacking in D. elongata , D. carinulata , and D. meridionalis ( Figs. 14, 17–19, 22–23 View FIGURES 14–18 View FIGURES 19–23 ). The EES of D. carinata only occasionally bears a lateral appendage ( Figs. 20 View FIGURES 19–23 — Artvin) or a lateral notch ( Fig. 20 View FIGURES 19–23 — Ashgabat). In D. sublineata , the spined area of the EES extends greater than or equal to 0.31 times (or greater than about one third) the length of the EES ( Figs. 16 View FIGURES 14–18 , 21 View FIGURES 19–23 , 48 View FIGURE 48 ; SL). In contrast, the spined area of the EES is confined to less than or equal to 0.16 times (or less than about one fifth) the length of the sclerite in D. elongata ( Table 3; Figs. 14 View FIGURES 14–18 , 19 View FIGURES 19–23 , 24 View FIGURES 24–28 , 48 View FIGURE 48 ). In D. sublineata , spines of the EES are often irregularly spaced along the blade with conspicuous gaps ( Fig. 21 View FIGURES 19–23 ). In D. carinulata and D. meridionalis , spines along the EES are usually evenly and closely spaced along the blade ( Figs. 22–23 View FIGURES 19–23 ). The EES of D. meridionalis additionally bears a hooked apex that is absent in D. sublineata .
Measurements. See Tables 2 and 3.
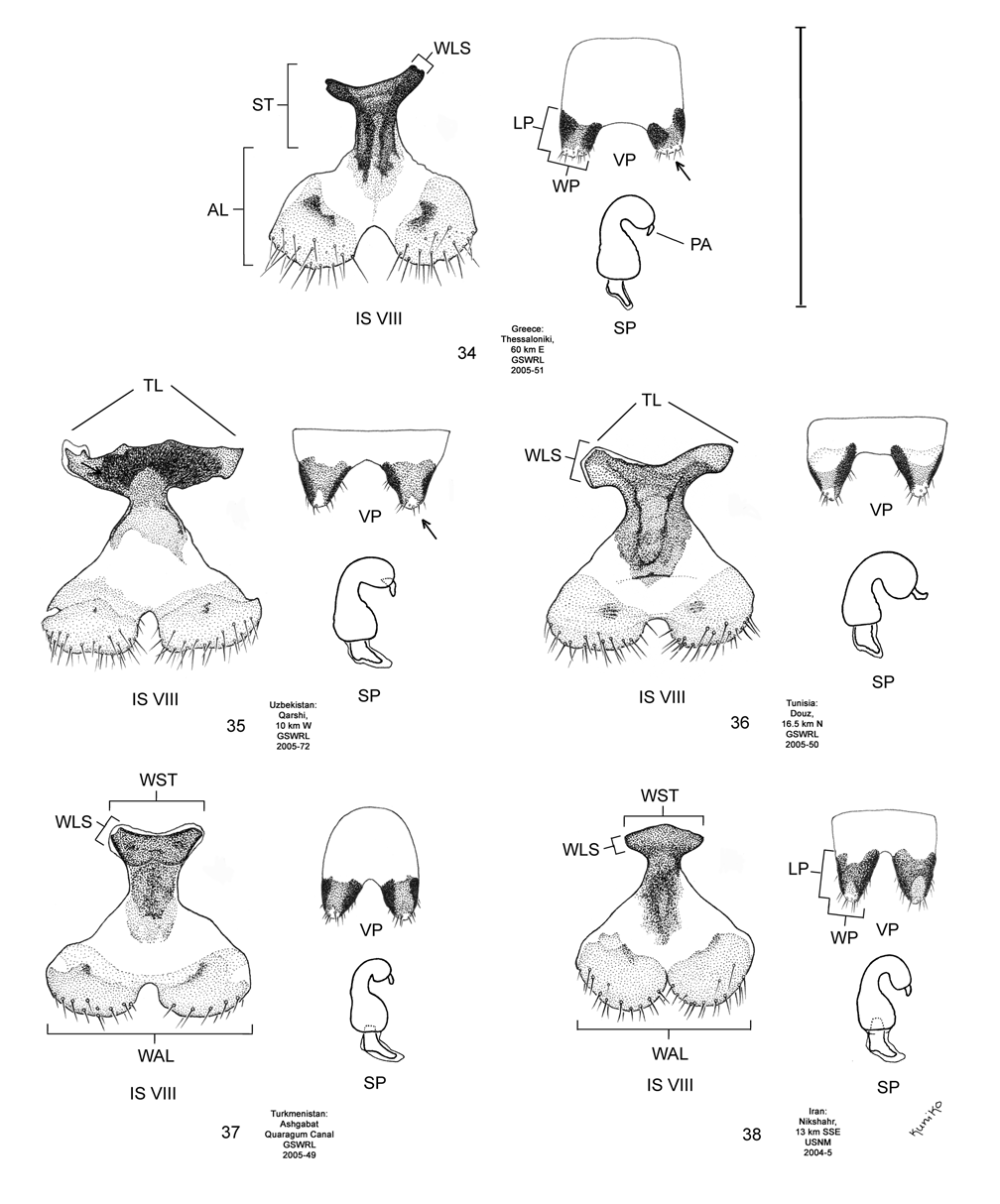
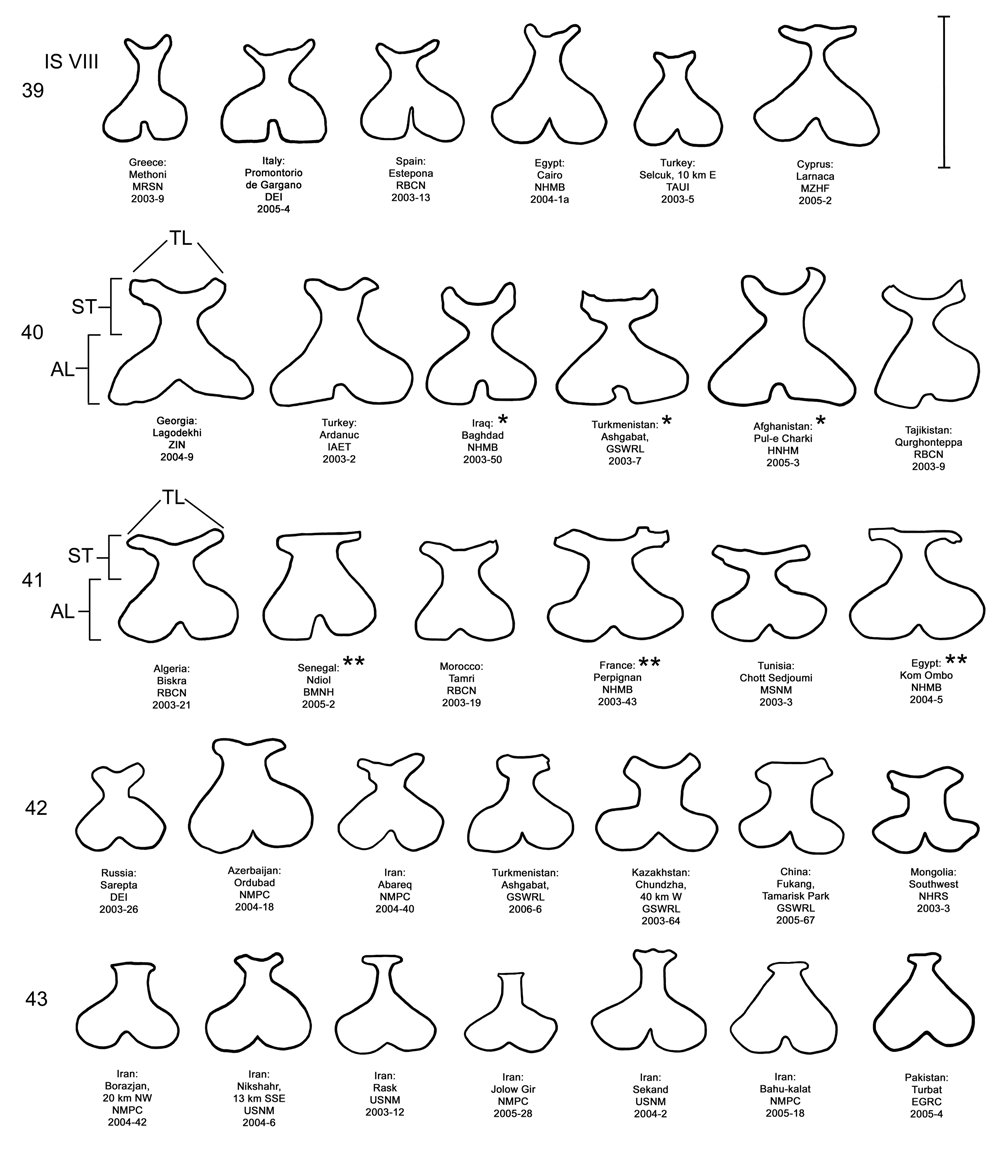
Female. Genitalia. Female D. sublineata may be distinguished from all other members of the D. elongata group except D. carinata by their triangulate vaginal palpi (VP) that are wider than long with a width to length ratio (LP/WP) of 0.46–0.85 (n = 19) ( Fig. 36 View FIGURES 34–38 ; Table 4). In contrast, the vaginal palpi are broadly rounded with a length to width ratio of 0.94–1.36 in the allopatric D. carinulata ( Fig. 37 View FIGURES 34–38 ) and D. meridionalis ( Fig. 38 View FIGURES 34–38 ; Table 4). The vaginal palpi are also broadly rounded in D. elongata ( Fig. 34 View FIGURES 34–38 ). In addition, the width of the widest lobe of the stalk (WLS) of internal sternite VIII (IS VIII) is often larger in D. sublineata (range 0.08–0.18 mm; Fig. 36 View FIGURES 34–38 ) compared to D. elongata (range 0.06–0.11 mm; Fig. 34 View FIGURES 34–38 ). Some female D. sublineata can be distinguished from D. carinata by having the tips of the lobes (TL) of the stalk of IS VIII either not curved or curved outward toward the apical lobe and the tips either rounded or quadrate ( Fig. 41 View FIGURES 39–43 – Ndiol, Perpignan, Kom Ombo), a state never found in D. carinata . Some D. carinata can be distinguished from D. sublineata by having the tips of both lobes of the stalk of IS VIII strongly curved inward at the tip and the tips either pointed or rounded ( Fig. 40 View FIGURES 39–43 – Baghdad, Ashgabat, and Pul-e Charki). Female D. sublineata and D. carinata with at least one lobe of the stalk of IS VIII curved only slightly inward ( Figs. 35–36 View FIGURES 34–38 , 40 View FIGURES 39–43 —Lagodekhi and Ardanuc, 41—Biskra and Tamri) are indeterminable to species, except on the basis of geographic distribution outside of the area of known sympatry in Iraq.
Measurements. See Tables 2 and 4.
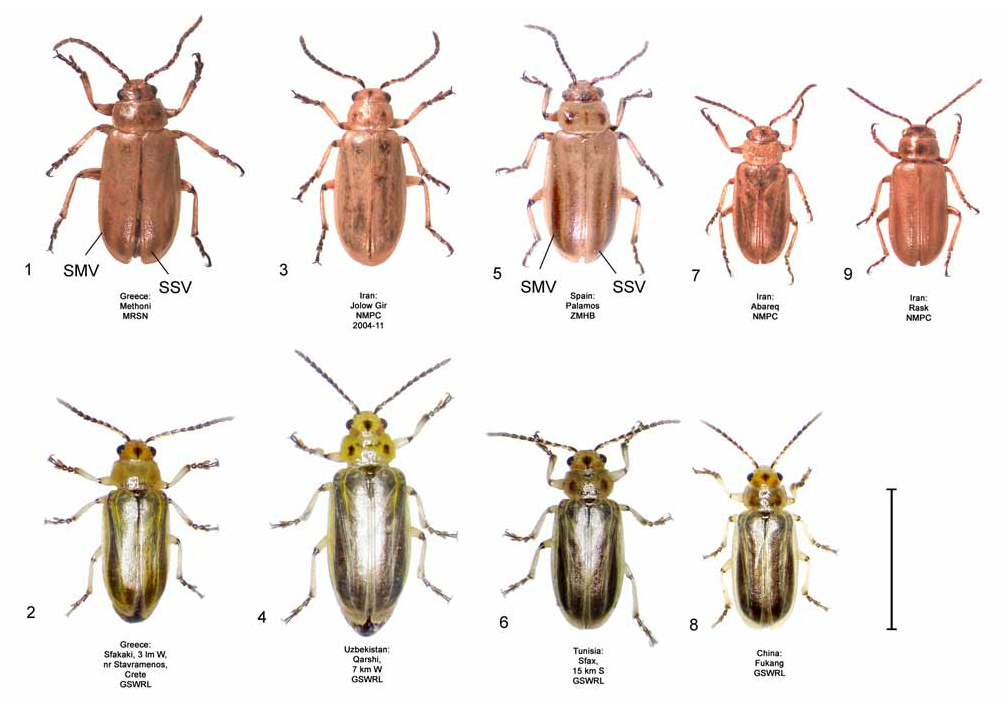
Coloration. In D. sublineata , subsutural and submarginal elytral vittae are often present, extending well into the basal half of the elytra ( Fig. 5 View FIGURES 1–9 ) and live specimens are tannish-yellow in hue, lacking any greenishyellow tinting ( Fig. 6 View FIGURES 1–9 ). This is in contrast to D. elongata in which the elytral vittae, if present, are confined to the apical half of the elytra ( Fig. 1 View FIGURES 1–9 ) and in which live specimens possess an olivaceous hue from greenishyellow tinting ( Fig. 2 View FIGURES 1–9 ). Diorhabda carinata differs from D. sublineata in more often lacking elytral vittae ( Fig. 3 View FIGURES 1–9 ) and in having some degree of greenish-yellow tinting in veins of the elytra ( Fig. 4 View FIGURES 1–9 ). Living D. sublineata ( Fig. 6 View FIGURES 1–9 ) and D. carinulata ( Fig. 8 View FIGURES 1–9 ) are very similar in coloration, and Chen (1961) notes the similar appearance of dead D. carinulata (as D. e. deserticola ) and D. sublineata (as D. e. ab. sublineata ).
Type material. Specimens from Lucas’s collections, which should include the type specimen(s) for D. sublineata , should be found at MNHN ( Groll 2006). The curator at MNHN communicated intent to inform us of the status of the type material and perhaps lend it for examination, but after four years, we have not heard of the status of the Lucas type material. Once it can be ascertained that the type material is lost, a neotype should be designated using a dissected male specimen from near the type locality of Annaba, Algeria . We studied the original description by Lucas (1849), possibly based on several individuals he stated were collected at Annaba, with his accompanying color habitus illustration. We also studied specimens from the broad vicinity of the type locality in Algeria and Tunisia . The location of type specimens for D. e. ab. bipustulata Normand (1937) is unknown and we studied the original description and specimens from the vicinity of the type locality of Kairouan, Tunisia .
Material examined. 89♂♂ dissected (diss.), 51♀♀ diss., 170♂♂, 191♀♀, 9 unsexed specimens. ALGERIA: 2♀♀ diss., [specific location not given], Reitter, NMPC [2004-21, 25]; 1♂, [specific location not given], Uyttenboogaart, Coll. Dr. D.L. Uyttenboogaart, ZMAN; 1♂ diss., 2♂♂, 3♀♀, Abadla [31.0167°N, - 2.7333°W], Kouril, P5/46/62, 4358a, NMPC [2004-03]; 1♂ diss., 6♀♀, Abadla, V. Zoufal, Kouril, P5/46/62, 4358a, D. elongata a. sublineata [ ZMHB], NMPC [2004-14], ZMHB [1♀]; 1♀, Abadla, ZMHB; 1♂ diss., 7♂♂, 8♀♀, Alger [36.76305°N, 3.05055°E], environs, Ch. Lallemam, Diorhabda elongata var. carinata det V. Laboissière 1939 [8♂♂, 8♀♀], D. e. var. sublineata det V. Laboissière 1939 [1♀], IRSNB [2006-02]; 1♂, Algir [Alger], coll[ection] Reitter, D. elongata, HNHM ; 1♂ diss., Argel [Alger], St. Perez Arcas, Galeruca elongata, MNMS [2003-01]; 1♂ diss., Biscra [Biskra; 34.85°N, 5.73333°E], Diorhabda elongata det I.K. Lopatin, Coll. Lichtnekert, HNHM [2003-03]; 1♂ diss., 1♂, 2♀♀, Biskra, environs, Obenb., NMPC [2004- 22]; 1♂ diss., 1♀ diss., 2♂♂, 6♀♀, Biskra, C.J. Dixon, Museum Lieden, RBCN [2003-08, 21]; 1♂ diss., 2♀♀ diss., 2♂♂, 1♀, Biskra, G.C. Champion, G.C. Champion Coll. B.M. 1927–409, BMNH [2003-03, 2003-11, 2005-01]; 1♀ diss., 1♀, Biskra, 1-III-1931, Dr. R. Meyer, NHMB [2005-08]; 2♀♀ diss., 3♀♀, Biskra, 30-III- 1985, M. Bergeal, MBPF [2003-01, 02]; 1♂, 3♀♀, Biskra, P. Jolivet, IRSNB; 1♀, Biskra, J. Sahlb., MZHF; 1♂ diss., Blidah [Blida; 36.41667°N, 2.828889°E], V-1867, H. Clark, 65-56, BMNH [2003-08]; 1♀ diss., Boghari [Boghni; 36.5422°N, 3.9531°E], V-1897, Dr. A. Chobaut, NMPC [2004-01]; 1♀ diss., Qued Hamman [Oued Hammam (Mascara State)], 10 km south Perregaux [Mohammadia] [35.50806°N, 0.040833°E], 9-V-1964, Eckerlein, Coll[ection] K.H. Mohr, DEI [2005-05]; 1♂ diss., 8♂♂, 13♀♀, Teniet el Had [Theniet el Had] to Affreville [Khemis Miliana] [app. location; 36.13950°N, 2.20749°E], Dr. Vauloger, D. elongata det. Le Moult, IRSNB [2006-07]; EGYPT: 5♂♂, 1♀, [specific locality not given], Reitter, D. sublineata det. V. Laboissière 1913 [ HNHM], NMPC [4♂♂, 1♀], HNHM [1♂]; 1♂, [specific locality not given], col[lection] Mus Murray, Fry coll[ection] 1905.100, Galleruca costipennis Dej cat [Dejean catalogue; nomen nudum; Dejean 1837, p. 377], BMNH; 2♂♂, [specific locality not given], ZIN; 1♂ diss., Cairo [Al Qahirah; 29.98333°N, 31.13333°E], Dachor, 29-I-1933, Schatzm. Koch., [D. e. ab.] sublineata det. Burlini 1966, MSNM [2003-17]; 1♂ diss., 1♂, 1♀, Cairo [Al Qahirah], Pyramidi, 3-X-1933, W. Wittmer, D. e. ab. sublineata det. Burlini 1966 MSNM [2003-02]; 10♂♂ diss., 3♀♀ diss., 43♂♂, 50♀♀, Com Ombo [Kom Ombo; 24.45°N, 32.93333°E], XI-1954, D. e. ab. sublineata det. J. Bechyne 1951, H. Hofbrauer, NHMB [2003-01, 04, 08, 2004-05, 06, 07, 08, 09; 2008-01, 02, 03, 04, 05]; 1♂ diss., 9 specimens, Dakhla Oasis [25.50722°N, 28.94722°E] MUT, 10-XII-1977, R.T. Simon Thomas, ZMAN [2008-11]; 1♂ diss., 1♂, 1♀, Dakhla Oasis, 4-III-1995, G. Strauss, RBCN [2003-02]; 1♂ diss., 3♂♂, 1♀, Ezbet El Nakl [Izbat an Nakhl; 30.15°N, 31.31666°E], 22-VIII-1933, W. Wittmer, [D. e. ab.] sublineata det. Burlini 1967, MSNM [2003-10]; 1♀ diss., 2♀♀, Fajum [Al Fayyum; 29.30778°N, 30.84°E], U. Sahlb., [no.] 610, MZHF [2003-01]; 1♀ diss., Hawara [pyramid; 29.27389°N, 30.90139°E], 2-V-1904, D. elongata det. L. Burgeon, D. e. var. sublineata det. V. Laboissière 1939, L. Burgeon Collection, IRSNB [2006-06]; 1♂ diss., 1♀, Heluan [Helwan des Bains; 29.85°N, 31.3333°E], 18-IX-1957, dr. Gozmány, in desert on Tamarix, Exc. Egypt Mus. Nat. Hung., D. e. var. sublineata det K. Lopatin, HNHM [2003-07]; 1♀ diss., Ismailia [30.5833°N, 32.2667°E], II, F. Lotte, USNM [2003-17]; 1♂, 2♀♀, Ismailia, F. Lotte, Février, ZIN; 4♂♂, 5♀♀, Ismailia, NMPC; 1♂ diss., 1♂, 5♀♀, Le Caire [Al Qahirah], NMPC [2004-45]; 1♂ diss., 1♂, Port Said [31.2667°N, 32.3°E], VI (June), F. Lotte, USNM [2003-19]; 1♀ diss., 1♀, Sakkarah, [ruins; 29.8457°N, 31.2104°E], Cairo (Gizeh), 19-II-1933, C. Koch, D. elongata det. Burlini 1966, MSNM [2008-03]; 1♂ diss., Siala [Silah; 29.3561°N, 30.9689°E], 21- VIII-1910, collection L. Burgeon, D. e. var. sublineata det. V. Laboissière 1939, IRSNB [2006-01]; 1♀ diss., Wadi Hoff [Ain Elwan Station; 29.86667°N, 31.31667°E], 20-V-1922, Alfieri, [no.] 1972, NHMB [2004-01]; FRANCE: 1♂ diss., 1♂, 3♀♀, Almanane [beach near Hyères; 43.0790°N, 6.1258°E], D. elongata det. V. Laboissière 1939, IRSNB [2006-03]; 1♂ diss., 11♂♂, 22♀♀, Camargue [region], Gallia, L. Puel, coll[ection] Leonhard [ DEI], coll. H.K. Mohr [ DEI], D. elongata [ DEI], DEI [2005-08; 4♂♂, 2♀♀], IRSNB [collection P. Jolivet; 7♂♂, 20♀♀]; 10 specimens, Ilis [37.8833°N, 21.3833°E], Olympia, Ellas, 2–3-X-1962, Ent. Exe. Zoöl. Mus., ZMAN; 2♂♂ diss., 2♂♂, 2♀♀, Le Lavandou [43.1322°N, 6.3645°E], Exped. Obenb., NMPC [2004-19, 2008-01]; 2♀♀, Le Lavandou, IV-1910, H. Desbordes, D. elongata det. Le Moult, IRSNB; 1♂ diss., 1♂, Le Barcares [42.78333°N, 3.03333°E], 19-V-1973, G.J. Slob, RBCN [2003-05]; 1♂ diss., Stes. Mairies [Les Saintes Maires, 43.45°N, 4.4333°E], Camargue, 11-13-IX-1951, H.H. Weber, NHMB [2003-19]; 1♀ diss., Les Stes.–Maries [Les Saintes Maires], 14-VII, W. Liebmann, DEI [2005-07]; 1♂ diss., 5♂♂, Marseille [43.3°N, 5.4°E], 16-VIII-1952, Hakan Lindb., MZHF [2004-01]; 1♂ diss., Montpellier [43.6°N, 3.833°E], Jentsson, tamarisci, Janis, DEI [2003-17]; 1♂ diss., 1♂, 1♀, Palavas [Palavas–les–Flots; 43.53333°N, 3.93333°E], [no.] 25-11, MBPF [2003-06]; 1♀ diss., Perpignan [42.68333°N, 2.88333°E], Pyr[ennes Mts.], Or[iental], IV-1953, J.V. Bechyné, NHMB [2003-43]; 1♂ diss., 3♂♂, 10♀♀, Salin–de–Giraud [43.4167°N, 4.7333°E], B du Rhône, Camargue, 12-IX-1970 [ RBCN 2003-17; 2♀♀ ZMAN], 14-IX-1970 [ ZMAN], C. van Nidek, D. elongata [ ZMAN], ZMAN, RBCN [2003-17]; 1♂ diss., Séveillé [geocoordinates not locatable], southern France, coll[ection] Bourgoin, D. elongata det. Le Moult, IRSNB [2006-05]; IRAQ: 1♂ diss., Baghdad [33.33861°N, 44.37722°E], IV-1936, Frey, NHMB [2003-31]; MOROCCO: 1♀ diss., Asni [31.25°N, - 7.98333°W], 1,000 m [elev.], High Atlas [Mts.], 9-VII-1993, Stn. Sammlung Dieter Siede, D. elongata det. R. Beenen 1995, RBCN [2003-10]; 2♂♂ diss., Driouch [34.9833°N, - 3.3833°W], near Midar, Nador [Prov.], 24-VI-1992, J.M. Vela, D. elongata det. Vela 1992 [1♂ diss.], GSWRL [2003-36, 2005-78]; 1♂ diss., Essaouira environs [31.51305°N, - 9.76972°W], Ounara, 3-V-1983, S. Doguet, MBPF [2003-08]; 1♂ diss., Goundafa [Talaat n' Yakoub; 30.9833°N, - 8.15°W], 1,200 m [elev.], Haut Atlas [Grand Atlas Mts.], 15-30-IV-1933, Schwingenschus, NHMB [2003-18]; 1♂ diss., 2♀♀, Marrakesch [Marrakech; 31.6333°N, - 8.000°W], 15-V-1992, Liebegott, D. elongata det. Eber 1995 [1♂ diss., ab. bipustulata], ZMHB [2006-03]; 1♂ diss., 1♀, Marrekech, 21–23-V-1926, Lindberg, MZHF [2008-02]; 1♂ diss., 6♀♀, 2♂♂, Tacheddirt [31.1667°N, - 7.85°W] Schwingenschus, NHMB [2003-02]; 1♀ diss., Tamri [30.6978°N, - 9.8253°W], Kuste [coast], 31-VII-1993, Stuben, D. elongata det. R. Beenen 1995, RBCN [2003-19]; 2♂♂ diss., 3♀♀, 1♂, Tanger [Tangier; 35.78472°N, - 5.812778°W], Rolph, Galeruca elongata , tag 1444, DEI [2003-06, 23]; 1♀ diss., Tanger [Tangier], 25–29-IV-1926, Lindberg, 889, MZHF [2008-01]; 1♂ diss., Tanger [Tangier], 1897, MNMS; 2♂♂ diss., 1♀ diss., 5♂♂, 3♀♀, Tarondant [Taroudannt], 12 km east [30.52268°N, - 8.759°W], 11-V-1958, C.J. Davis, on Tamarix sp. , #83, 58-9590, 58-9591, 58-9592, USNM [2003-01, 02, 03]; 1♂ diss., 1♂, Taroudant [Taroudannt; 30.4833°N, - 8.8667°W], 28-V-1985, G. Sama, MRSN [2003-04]; 1♂ diss., Taroudannt, Sous Valley, 18-IV-1990, Z. Kejval, D. elongata, EGRC [2005-06]; 1♂ diss., 3♀♀, Tiz-n-Test Pass [30.83333°N, - 8.33333°W], High Atlas [Mts.] 20-VI-1994, Szailles, RBCN [2003-11]; PORTUGAL: 1♂ diss., Lusitania [Portugal, specific locality not given], Reitter, ZMAN [2008- 16]; SENEGAL: 1♂ diss., specific locality not given, Mion, ZMHB [2006-06]; 2♂♂ diss., 1♀ diss., 8♂♂, 2♀♀, Ndiol [Ndiol Nar; 16.14778°N, - 16.31306°W], 25 km northeast St. Louis, 15-VIII-1979, P. Jolivet, on T. senegalensis , D. sp. nr. elongata det. S.L. Shute 1979, BMNH [2003-01, 02, 2005-02]; SPAIN: 1♀ diss., [specific locality not given], Lolobregato, NHMB [2003-05]; 2♂♂, 3♀♀, m[eridionalis; southern; specific locality not given], Minsmer, [18]99, ZMAN; 1♂, 1♀, [specific locality not given], L. Miller, coll. Ed. Everts, ZMAN; 1♂ diss., Almeria [36.8833°N, - 2.45°W], Tabernas Desert, 21-VII-1965, La Greca, MRSN [2003- 03]; 2♂♂, Andalucia [Comunidad Autonoma de Andalucia; specific locality not given]; coll[ection] Reitter, D. elongata, HNHM ; 1♂, 1♀, Andalusia [Andalucia], Baly coll[ection], Galeruca sublineata, BMNH ; 1♂ diss., Barcelona [41.3833°N, 2.1833°E], X-1940, F. Monros, USNM [2003-07]; 3♀♀ diss., Barcelona, Farola de Llobregat, 15-VII-1940, F. Monros Coll., USNM [2005-02, 05, 06]; 1♂ diss., Cabo de Gata [El Cabo de Gata; 36.7833°N, - 2.2333°W], 4-VI-1977, Hinterseher, D. elongata det. Erber 1985, D. elongata det. R. Beenen 1996, ZMHB [2008-03]; 1♂ diss., 5♂♂, Cordoba [37.83333°N, - 4.7667°W], Col del Sur Perez Arcas, G. elongata, MNMS [2004-03]; 1♀ diss., Galicia [Autonomous Community; specific locality not given; estimate approx. locality in Pontevedra Province, the only province of Galicia where Tamarix is noted ( Cirujano 1993): 42.0186°N, - 8.87510°E], [D.] elongata, NMPC [2005-31]; 2♀♀ diss., Halizia [Galicia Autonomous Community; specific locality not given], [D.] elongata, NMPC [2004-16, 2005-27]; 1♂ diss., Rambla del Grao, Quadix [37.3°N, - 3.1333°W], Granada, F.S. Pinero, JOSV68935, GSWRL [2005-77]; 1♂ diss., 3♂♂, Lorca [37.6667°N, - 1.7°W], Murcia, VIII-1943, G. Menor, MNMS [2004-02]; 1♂ diss., Madrid [40.4°N, - 3.6833°W], Montarco, Peris Torres, MNMS [2004-01]; 1♀ diss., 14♂♂, 14♀♀, Palamos [41.85°N, 3.13333°E], Catalonien, ZHMB [2003-06]; 1♂ diss., 4♂♂, 3♀♀, Playa de Aro [41.8167°N, 3.0667°E], 2-17- VII-1963, D. elongata det. L. Borowiec, MZLU [2005-04]; 1♀ diss., Sierra de Javalambre [Mt.; 40.1°N, - 1.0°W], Teruel, 1,600m [elev.], 25-VII-1965, La Greca, D. e. subsp. sublineata det. Daccordi 1971, MSNM [2003-04]; 1♂ diss., 2♂♂, 4♀♀, Tossa de Mar [Tossa; 41.7167°N, 2.9333°E], 29-VI to 19-VII-1968, Th. Palm, D. elongata det. L. Borowiec, MZLU [2005-03]; 1♂ diss., Totana [37.7667°N, - 1.5°W], Murcia, V- 1938, Balaguer, D. elongata det. Petitpierre, GSWRL [2003-05]; 1♂ diss., 1♂, Valencia [39.4667°N, - 0.3667°W], Maru'der, MNMS [2003-02]; 1♂ diss., 2♀♀, Vera [37.2500°N, - 1.8667°W], with Almira [Almeria], 4-VI-1977, brookside, D. elongata det. Steinhausen [1♂ diss.], D. elongata det. Eber 1985 [2♀♀], ZMHB [2006-02]; TUNISIA: 1♂ diss., Belvedere [36.82472°N, 10.18638°E], 25-IX-1929, A. Schatzmayer, [D. e. ab.] sublineata det. Burlini 1967, MSNM [2003-07]; 1♀ diss., Boughrara [33.5378°N, 10.6761°E], 9- IV-1977, S. Mahunka, D. elongata det. V. Tomov, No. 99, HNHM [2003-08]; 1♂ diss., 1♀ diss., 1♂, 1♀, Douz, 16.5 km north [33.6075°N, 9.005°E], 19-V-2000, A. Kirk, on Tamarix spp. , GSWRL [2003-42, 2005- 50]; 1♂ diss., 1♀ diss., Chott el Guetar [34.39°N, 8.84°E], [9.3 km southeast] Gafza [Gafsa], 200m [elev.], Lortess, 18-IV-1993, R. Regalin, D. elongata det. D. Regalin 1995 [♀], Coll[ection] D. Sassi [♀], GSWRL [2003-61], MRSN [2003-08]; 1♂ diss., 1♀ diss., 2♀♀, Chott Sedjoumi [36.765°N, 10.15055°E], 27-IX-1929, A. Schatzmayer, D. e. ab. sublineata det. Burlini 1967, MSNM [2003-01, 03]; 1♂ diss., Djedeida [Jedeida; 36.83111°N, 9.92416°E], 14-X-1929, A. Schatzmayer, [D. e. ab.] sublineata det. Burlini 1967, MSNM [2003- 05]; 2♂♂ diss., 1♂, 7♀♀, Marith, 18.7 km southeast on road C116, 33.5760°N, 10.4553°E, 20-V-2008, Javid Kashefi, on Tamarix sp. in desert wadi, shipment EBCLGR-JK-2008-004, New Mexico, Las Cruces, NMSU lab colony F4 generation, 8–27-X-2008, D. Guenther, voucher shipment GSWRL-2008-03, GSWRL [2008- 23, 24]; 1♀ diss., 2♀♀, Gabes [33.7425°N, 10.20567°E], Medenine road, 15-V-2000, A. Kirk, on Tamarix spp. , GSWRL [2003-49]; 2♂♂ diss., Gabes, beach at Hotel Chems [33.20530°N, 11.20970°E], 6-V-2007, J. Kashefi, Tamarix sp. (not T. aphylla ) (1♂), T. aphylla (1♂), shipment GSWRL-2007-01, GSWRL [2007-48, 49]; 1♂ diss., 3♂♂, 4♀♀, Kebili [33.70483°N, 8.942333°E], Tozeur side, 18-V-2000, A. Kirk, on Tamarix spp. , GSWRL [2003-43]; 2♂♂ diss., 4♀♀ diss., 1♂, 1♀, Sfax [34.7406°N, 10.7603°E], Gargour, Nahkia, 14- V-2000, A. Kirk, on Tamarix sp. , GSWRL [2003-09, 12, 50, 65, 76, 79]; 4♂♂ diss., 4♀♀ diss., Sfax, 15 km south [34.66°N, 10.67°E; not Tunis as in some shipping records], 30-IX-2002, A. Kirk, on Tamarix spp. , voucher USDA lab colony at Albany, California (22-I-2003, 2003, D. Bean), voucher USDA lab colony at Temple, Texas (1♂, 1♀, 26-VIII-2003, 5-IX-2003, L. Milbrath [nos. 1286, 1283]), shipment EIWRU-2002- 1008, GSWRL [2003-14, 15, 22, 23, 74, 75, 86, 87] [released in south Texas in 2005] [used in biological studies of Milbrath and DeLoach (2006a, 2006b), Milbrath et al. (2007), Herr et al. (in prep.), Bean and Keller (in prep.), and Thompson et al. (in prep.)]; 1♂ diss., Sfax, 15 km South [34.63980°N, 10.65280°E], 6-V-2007, J. Kashefi, Tamarix sp. (not T. aphylla ), shipment GSWRL-2007-01, GSWRL [2007-50]; 1♀ diss., Sfax, 17 km South [34.65°N, 10.66°E], 6-III-1995, Kirk & Sobhian, on Tamarix spp. , 9137, D. elongata det. I. Lopatin 1999, GSWRL [2004-15]; 1♂ diss., 2♂♂, Tozeur [33.92055°N, 8.13333°E], 14-XII-1928, Torre e Tasso, [D. e. ab.] sublineata det. Burlini 1967, MSNM [2003-06]; UNKNOWN COUNTRY: 1♂ diss., Pyrenneaen [not locatable, probably Pyrennes Mts., possibly France], Porrir, Scheider der Kelch, DEI [2003-12]; YEMEN: 2♀♀ diss., Yemen [specific locality not given], Arabia, Millingen [approx. location near Haraz Mts. ( Millingen 1874): 14.87820°N, 43.65640°E], Fry Coll. 1908.100, BMNH [2003-04, 05].
Distribution. General. Diorhabda sublineata ranges from Portugal, Spain and France to Morocco, Senegal, Algeria, Tunisia, Egypt, Yemen, and Iraq (Map 4). Chatenet (2002) reported Diorhabda was little common in France and Petitpierre (1988) reported it as infrequent along the northeastern Mediterranean coast of Spain. It is apparently of sporadic occurrence in southern Spain, where surveys of Tamarix in July 2005 revealed no Diorhabda (J. Sanabria and J. Vela, pers. comm.). But Hopkins and Carruth (1954) report Diorhabda as common in Huelva Province of southwest Spain. Boehm (1908) found D. sublineata to be the most common galerucine in Egypt and Pierre Jolivet (Paris, France, pers. comm.) collected specimens which we have dissected from a dense population in the thousands at Ndiol Nar, Senegal. Reports of D. e. var. sublineata ( Weise 1890) , D. e. ab. sublineata (Medvedev and Voronova 1977b, Medvedev 1982), and D. e. sublineata (Gressitt and Kimoto 1963a; Lopatin 1968, 1975, 1977b) from Mongolia or China should refer to D. carinulata . Further collections should reveal that the range of D. sublineata includes Western Sahara, Mauritania, Libya, Sudan, Saudi Arabia, and Kuwait and yield specific locations in Portugal, north Yemen, and the Pontevedra (Galicia) and Huelva provinces of Spain. Tamarix aphylla and the probable host, T. nilotica , are found in east Africa in Ethiopia and Somalia ( Baum 1978), where D. sublineata may also occur. Two Tamarix spp. are endemic to southern Africa (in Angola, Namibia and South Africa; Baum 1978), where surveys for D. sublineata should be made.
Confirmed Records. We have dissected specimens from (see Material Examined) and confirmed the presence of D. sublineata (as D. e. var. sublineata , D. e. ab. sublineata , and D. sublineata ) in the following countries with previous literature records (Map 4): Algeria ( Lucas 1849, Peyerimhoff 1926), Egypt ( Boehm 1908, Alfieri 1976), Tunisia ( Normand 1937), and France ( Laboissière 1934).
New Records. We dissected specimens of D. sublineata from the following countries for which we find no previous specific reports of this species in the literature: Portugal, Spain, Morocco, Senegal, Iraq, and Yemen. With the exception of Yemen, we find past reports of D. elongata from these countries that should refer, at least in part, to D. sublineata (see above synonymy).
Specimens at BMNH from Yemen with a label of “Millingen” were probably collected circa 1873 on a journey by Dr. Charles Millingen to north Yemen where he noted tamarisk in lowlands surrounding the Haraz Mountains ( Millingen 1874).
Unconfirmed Records. No country records of D. sublineata remain unconfirmed. On the basis of evidence discussed below, we accept as D. sublineata several locality records of Diorhabda from France, Spain, and North Africa. Diorhabda sublineata has been released in the United States (Texas), but establishment is not yet confirmed (Map 7, see Potential in Tamarisk Biological Control below for additional details).
Specimens of D. sublineata were dissected from all 11 available locations from France. We examined a specimen of D. sublineata from France (IRSNB) with an identification label of D. e. var. carinata made by Laboissière in 1939. Therefore, we consider Laboissière’s (1934) report of D. e. var. carinata in France as D. sublineata , although D. elongata may also be present. Of 14 locations in Spain, D. sublineata was dissected from 13 of these while D. elongata was dissected from only one. Therefore, we believe Hopkins and Carruth (1954) found D. sublineata , rather than D. elongata , to be common in the Huelva Province of southwest Spain. Torres Sala (1962) reported D. sublineata (as D. elongata ) from Comunidad Valenciana, Spain. Petitpierre (1988) found D. sublineata (as D. elongata ) was infrequent along the northeastern coast of Spain.
Specimens of D. sublineata were dissected from all 40 specific localities available from North Africa. D. elongata was also present at only one specific locality, Al Qahirah (Cairo), Egypt, and one general locality, Algeria, from a series predominated by D. sublineata . Consequently, we accept as D. sublineata all of the 23 unconfirmed North African collection records of D. sublineata ( Lucas 1849; see also below Discussion- Taxonomy), D. e. ab. sublineata ( Normand 1937, Alfieri 1976), D. e. ab. bipustulata ( Normand 1937), and D. elongata ( Peyerimhoff 1926; Normand 1937; Jolivet 1967; A. Kirk; USDA/ARS, Montferrier-sur-Lez, France, retired, pers. comm.; S. Doguet, pers. comm.). Weise (1925) reported that an expedition to Anglo- Egyptian Sudan collected D. sublineata (as D. elongata ) from Halwan (= Heluan), Egypt, a location from which we dissected D. sublineata . This record was apparently incorrectly cited by Laboissière (1934) as a collection from Sudan, and this error was followed by Ogloblin (1936).
Below are listed 25 unconfirmed locality records that we assign to D. sublineata (Map 4):
ALGERIA: Hippône [Annaba; 36.9000°N, 7.7667°E] ( Lucas 1849); Djamaa [33.5333°N, 6.0000°E], on Tamarix boveana (as T. bounopaea ), III; Maison Carree [El Harrach; 36.7203°N, 3.14500E], on Tamarix africana, IX ( Peyerimhoff 1926) ; Aurès Mountains, Beni Imloul Forest, Sidi Ali [=Ain Sidi Ali; 35.2833°N, 5.4667°E], 14-VI-1981, S. Doguet, on Tamarix ; Biskra, Oued Beraze, 24-VI-1981, S. Doguet, on Tamarix ; Guelma, Djebel Taya [36.507°N, 7.0828°E], 1-II-1968, S. Doguet, on Tamarix ; Kabylie, El Adjiba [36.3258°N, 4.1503E], VI-1959, J.C. Bourdonné; Nemanchas Mountains, Khangat Sidi Nadji [Sidi Naji; 34.8167°N, 6.7000°E], 24-VI-1981, S. Doguet, on Tamarix (S. Doguet, pers. comm.); EGYPT: Asyut [27.1828°N, 31.1828°E], IX; Cairo vicinity, V-X [ D. sublineata dissected from same location]; Helwan [Halwan], wadis northeast, V ( Alfieri 1976); Heluan [Halwan] [ D. sublineata dissected from same location] ( Weise 1925); Minya, IX [Al Minya; 28.1194°N, 30.7444°E]; Siala [Silah; 29.3561°N, 30.9689°E], VI [ D. sublineata dissected from same location]; Sinnuris [29.4167°N, 30.8667°E], VI ( Alfieri 1976, on Tamarix spp. ); FRANCE: Narbonne [43.1833°N, 3.0000°], D. e. var. carinata ( Laboissière 1934) ; MOROCCO: Essaouira, Ounara [31.5336°N, 9.5536°W], 3-V-1983, S. Doguet, on Tamarix ; Tamelelt [Tamelelt Jdida; 31.8189°N, 7.5089°W], Marrekech [ D. sublineata dissected from same location], 11-V-1983, S. Doguet, on Tamarix (S. Doguet, pers. comm.); Melilla area [33.3881°N, - 7.14500°E], on Tamarix sp. ; Sidi Mouna el Harati [Sidi Moussa el Harrati shrine; 34.0800°, - 5.9600°E], on T. africana ( Jolivet 1967) ; South Essaouira, Tamri [30.6780°N, 9.8253°W], 4-V-1983, S. Doguet, on Tamarix (S. Doguet, pers. comm.); SPAIN: Empúries [Ampurias; 42.1333°N, 3.1167°E] ( Petitpierre 1988); Huelva [Province in southwest Spain; specific locality not given; app. locality: 37.25830°N, - 6.9508°E], Tamarix gallica (Hopkins and Carruth 1954) ; Torroella de Montgri [42.0333°N, 3.1333°E]; Valls [41.2833°N, 1.2500°N] ( Petitpierre 1988); TUNISIA: Le Kef [El Kef; 36.1822°N, 8.7147°E], Kairouan [35.6744°N, 10.1017°E], Medjez–el–Baba [Majaz al Bab; 36.6500°N, 9.6167°E] ( Normand 1937); 1 spmn., Douz, 20.2 km N, 33° 37' 52" N, 8° 59' 58" E [33.6311°N, 8.9994°E], 19-V-2000, A. Kirk, Tamarix aphylla (Alan Kirk, USDA/ARS, Montpellier, France, retired, pers. comm.).
D. sublineata × D. elongata Hybrid Morphology. All six studied adult male D. sublineata × D. elongata laboratory hybrid F1 and F2 progeny ( Figs. 46–47 View FIGURES 44–47 ) obtained from D.C. Thompson and B. Peterson ( NMSU) can be distinguished from parental pure lines ( Figs. 44–45 View FIGURES 44–47 ) by a variety of anomalous hybrid genitalic character combinations in the endophallic sclerites. These diagnostic character combinations are not seen in laboratory pure lines of D. sublineata and D. elongata ( Figs. 44–45 View FIGURES 44–47 ) or in field collected material anywhere in the Palearctic, including areas of marginal sympatry for D. sublineata and D. elongata such as Portugal, Spain and Egypt ( Figs.14, 16 View FIGURES 14–18 , 19, 21 View FIGURES 19–23 , 24, 26 View FIGURES 24–28 , 29, 31 View FIGURES 29–33 ). Examples of anomalous character combinations in hybrids include: (1) length of blade ( LB) of elongate endophallic sclerite ( EES) is intermediate between the maximum length of blade for D. elongata (0.44 mm), and the minimum length of blade for D. sublineata (0.52 mm) ( Figs. 46–48 View FIGURES 44–47 View FIGURE 48 , NMSU 2006-1, 2); (2) length of spined portion ( SL) of EES is intermediate between the maximum spined length for D. elongata (0.18 mm) and the minimum spined length for D. sublineata (0.38 mm) ( Figs. 46–48 View FIGURES 44–47 View FIGURE 48 , NMSU 2006-3, 4, 5); (3) length of blade of EES falls within the range for D. sublineata , but length of spined portion of the EES falls within the range for D. elongata ( Figs. 47–48 View FIGURES 44–47 View FIGURE 48 , NMSU 2006-6), or is intermediate between the species ( NMSU 2006-3); (4) length of blade of EES falls within the range for D. sublineata , but connecting endophallic sclerite ( CES) is absent as in D. elongata ( Figs. 47–48 View FIGURES 44–47 View FIGURE 48 , NMSU 2006-3, 4, 6); (5) palmate endophallic sclerite ( PES) is broadly rounded with subdistal spines as in D. elongata , but length of blade of EES falls within the range of D. sublineata ( Fig. 47 View FIGURES 44–47 — NMSU 2006-4); and (6) PES is truncate serrate with more than two spines being distal as in D. sublineata , but length of blade of EES falls within the range of D. elongata ( Fig. 47 View FIGURES 44–47 — NMSU 2006-5). All hybrids examined lack the CES ( Figs. 46–47 View FIGURES 44–47 ) which is indicative of D. sublineata ( Fig. 45 View FIGURES 44–47 ). Of the six D. sublineata / D. elongata hybrids illustrated, most bear the external coloration and pattern of elytral vittae found in D. elongata , and only one hybrid ( Fig. 47 View FIGURES 44–47 — NMSU 2006-5) had submarginal and subsutural elytral vittae extending into the base of the elytra as is sometimes found in D. sublineata . The morphologies of later generation hybrids or various types of backcross hybrids are not characterized.
Many of the male D. sublineata × D. elongata hybrids possess genitalic abnormalities in the form of conspicuous abnormal sclerites ( AS) of varying number, size, shape and location on the endophallus ( Figs. 46–47 View FIGURES 44–47 ). Abnormal sclerites are not seen or are small and inconspicuous in parental pure lines ( Fig. 44–45 View FIGURES 44–47 ). Externally normal appearance with genitalic abnormalities is commonly observed in interspecific hybrids between some sibling species of Drosophila fruit flies (e.g., Hollocher et al. 2000). Abnormal endophallic sclerites in D. sublineata × D. elongata hybrids are evidence of genetic incompatibilities and possibly some level of reduced hybrid fitness and postzygotic reproductive isolation.
Discussion. Taxonomy. Galeruca sublineata Lucas (1849) (as Galleruca sublineata ) was described from Hippône (= Annaba), Algeria, and synonymyzed under G. elongata by Reiche and Saulcy (1858). Weise (1890) proposed D. elongata var. sublineata as extending in range from North Africa to Mongolia. Weise (1924) later proposed the aberration D. e. ab. sublineata and this has been followed by several taxonomists ( Ogloblin 1936, Normand 1937, Alfieri 1976, Warchalowski 2003). Chen (1961) excluded D. e. ab. sublineata from Mongolia and included the Mongolian population under the new subspecies D. e. deserticola described from western China. Apparently unaware of Chen’s designation, Gressit and Kimoto (1963) proposed the subspecies D. e. sublineata as occurring from Mongolia and western China west to North Africa. The catalogue of Lopatin et al. (2004) lists D. e. sublineata as a junior synonym of D. e. elongata .
In order to characterize the genitalia of G. sublineata , we examined specimens that matched the species description of G. sublineata from Algeria and Tunisia, in the broad vicinity of the type locality of Annaba, Algeria. In the color habitus illustration accompanying the species description for G. sublineata by Lucas (1849, Plate 44, Fig.8 View FIGURES 1–9 ), the submarginal and subsutural elytral vittae extend well into the basal half of the elytra (as in Fig. 5 View FIGURES 1–9 ). This characteristic of the elytral vittae can be used to distinguish G. sublineata from D. elongata . In D. elongata , the elytral vittae are often absent, as in the color habitus illustration with the species description by Brullé (1832, Plate 44, Fig. 10 View FIGURES 10–13 ), and, when present, the elytral vittae are confined to the apical half of the elytra ( Fig. 1 View FIGURES 1–9 , see D. elongata - Discussion- Taxonomy above). From 12 locations in Algeria and Tunisia, we selected ten male and seven female specimens matching the description of G. sublineata with elytral vittae extending well into the basal half of the elytra. We are confident that these 17 specimens represent G. sublineata as described by Lucas (1849).
We studied the genitalia in the 17 above specimens matching G. sublineata from Algeria and Tunisia. In the ten males, the endophalli bear a combination of five characteristics distinguishing them from those of D. elongata (characterized above) and the holotypes of D. carinulata , D. meridionalis , and D. carinata ( Figs. 18–19 View FIGURES 14–18 View FIGURES 19–23 of Berti and Rapilly 1973) (see Male- Genitalia above; Figs. 14–33 View FIGURES 14–18 View FIGURES 19–23 View FIGURES 24–28 View FIGURES 29–33 ). In the seven females matching G. sublineata , the vaginal palpi were triangulate and narrowly rounded, as in D. carinata , and distinct from the broadly rounded vaginal palpi of D. elongata . The distinct male and female genitalic characters of specimens matching G. sublineata are strong evidence of reproductive isolation from other species in the group, and especially from the two species with which it is marginally sympatric, D. elongata and D. carinata (see Biogeography below; Map 1, Table 8). Therefore, we remove G.sublineata from synonymy with D. elongata and restore the species D. sublineata (Lucas) REVISED STATUS. Although D. sublineata is allopatric with D. carinulata and D. meridionalis , their status as reproductively isolated is supported by the number of diagnostic genitalic characters separating D. sublineata from D. carinulata and D. meridionalis (6–8 characters) being greater than the number of characters separating the moderately sympatric D. carinata from D. carinulata (5 characters) ( Table 5). Further evidence for reproductive isolation between D. sublineata and D. elongata is also found in previously discussed differences in component ratios of putative aggregation pheromones and reduced F2 hybrid egg viability. The elytral vittae of D. sublineata can resemble that of D. elongata in being confined to the apical half of the elytra or entirely lacking. The color habitus illustration of “ D. elongata ” from southern Europe in Plate 29, Figure 7 View FIGURES 1–9 of Chatenet (2002), with the elytral striping extending through the entire basal half of the elytra, is actually of a specimen of D. sublineata .
If D. sublineata interbreeds as a subspecies with D. elongata where these species are marginally sympatric in Portugal, Spain and Egypt, we would expect intermediate hybrid forms to be evident in areas of range contact as seen in other chrysomelid subspecies, such as Diabrotica virgifera virgifera and D. v. zeae Krysan and Smith ( Krysan et al. 1980) . For example, we would expect hybrid characteristics such as varying degrees of development of the connecting endophallic sclerite and the lateral appendage of the palmate endophallic sclerite and intermediate lengths in the blade of the elongate endophallic sclerite. Diagnostic hybrid characteristics are found in examined laboratory produced D. sublineata × D. elongata hybrids ( Figs. 46–47 View FIGURES 44–47 ; see D. sublineata × D. elongata Hybrid Morphology above). However, we find no specimens with diagnostic hybrid characteristics in field collections of 157 male D. elongata and 89 male D. sublineata . These include 37 specimens from the areas of range overlap; 6 male D. elongata in Egypt, ( Figs. 19 View FIGURES 19–23 , 24 View FIGURES 24–28 , 29 View FIGURES 29–33 — Cairo), Portugal ( Fig. 19 View FIGURES 19–23 — Portugal), and Algeria ( Fig. 19 View FIGURES 19–23 — Algeria) and 42 male D. sublineata in Egypt ( Figs. 21 View FIGURES 19–23 , 26 View FIGURES 24–28 , 31 View FIGURES 29–33 — Cairo), Algeria ( Fig. 21 View FIGURES 19–23 — Algiers), Spain ( Fig. 21 View FIGURES 19–23 — Valencia), and Portugal. The lack of intermediate hybrid forms in areas of sympatry is firm evidence of reproductive isolation between D. elongata and D. sublineata , and we are confident that D. sublineata is not a subspecies of D. elongata .
The diagnostic characters separating D. sublineata from D. elongata comprise three discrete and two near discrete male genitalic characters and one discrete female genitalic character for a total of six diagnostic genitalic characters, four of which are discrete (see keys and Table 5). In comparison, a total of five discrete genitalic characters are diagnostic in distinguishing the moderately sympatric species D. carinata and D. carinulata ( Table 5). Even if D. elongata and D. sublineata were allopatric rather than marginally sympatric, their degree of divergence in genitalic characters is similar to that found in the related sympatric species pair D. carinata / D. carinulata (see also D. elongata group Stenophenetic Analysis, Fig. 49 View FIGURES 49–50 ), further justifying their status as separate species (see Helbig et al. 2002).
As noted with other members of the D. elongata group, we find that the external characters that have been used in separating D. sublineata from other sibling species are inadequate and that genitalic characters must be used. Weise (1890) proposed that two sharply defined stripes on the elytra (elytral vittae) that joined apically on D. sublineata (as D. e. var. sublineata ) is a distinguishing character from D. elongata . The use of this character as diagnostic has been followed by Laboissière (1934) and Warchalowski (2003). However, apically joined elytral vittae variably occur in both D. sublineata and D. elongata . Chen (1961) noted that the ventral tarsal pubescence was generally distributed in D. e. ab. sublineata , as opposed to being medially absent (leaving a median glabrous area) in D. e. deserticola . However, we found that the pattern of ventral tarsal pubescence in specimens of D. sublineata from North Africa is the same as that described by Chen for D. e. deserticola , and regard this character as unsuitable for taxonomic diagnosis. We have dissected specimens of D. sublineata that were misidentified by taxonomists using external diagnostic characters as D. elongata from five countries and D. e. var. carinata from Algeria (see Material Examined).
We examined four specimens (from ZMHB) with old hand-written determination labels of D. e. var. sublineata from Weise’s collection that were collected by Potanin from Mongolia. All four of these specimens are D. carinulata . These specimens are probably from the same series listed by Weise (1890) as D. e. var. sublineata and collected by G.N. Potanin from central Mongolia. All material we have examined from Mongolia and China are conspecific with D. carinulata (discussed below), and D. sublineata is absent from these areas, the easternmost occurrence of D. sublineata being in Baghdad, Iraq (Map 1).
Normand (1937) described the aberration D. elongata ab. bipustulata Normand from Kairouan, Tunisia in the area where D. sublineata is common (Maps 1 and 4). This aberration is distinguished by two dark spots near the base of the elytra on either side of the scutellum. We saw this uncommon color variant in one specimen (from Morocco) of over 150 examined specimens of D. sublineata . It was also seen in six specimens (from Greece and Cyprus ) of 200 examined specimens of D. elongata , two specimens (from Ash Sharqat, Iraq) of over 150 examined D. carinata , and one specimen (from 74–164 km NW Turpan, China) of over 300 examined D. carinulata . Diorhabda elongata apparently is rare in Algeria and probably rarely occurs, if at all, in Tunisia, from which all 17 dissected specimens from all 12 available locations were D. sublineata . Therefore, we consider D. e. ab. bipustulata as a junior synonym of D. sublineata NEW SYNONYMY.
Diorhabda sublineata and D. carinata are the most morphologically similar species in the D. elongata group, and we considered the possibility that D. sublineata might be a subspecies of the earlier described D. carinata . The only character for consistent separation of D. sublineata from D. carinata is the presence of the connecting endophallic sclerite in the male ( Figs. 16 View FIGURES 14–18 , 21 View FIGURES 19–23 , 26 View FIGURES 24–28 , 31 View FIGURES 29–33 ). This connecting sclerite is not a trivial character in that it influences the three dimensional configuration of the inflated endophallus. In D. sublineata killed and preserved while copulating, the inflated endophallus is bent at the connecting sclerite and this is not seen in inflated endophalli of D. carinata . If D. sublineata and D. carinata were interbreeding subspecies, we should have observed intermediate forms in the critical distinguishing morphological character of the presence or absence of the connecting endophallic sclerite. Intermediate forms should increase along a geographic cline approaching the point of range contact in Iraq. Specifically, we should have observed a transition from the fully developed connecting endophallic sclerite of D. sublineata to increasing incidence of faint lines in place of a connecting endophallic sclerite, to the total lack of connecting endophallic sclerite found in D. carinata . From Baghdad, Iraq, we examined a single male D. sublineata with a normal connecting endophallic sclerite ( Fig. 21 View FIGURES 19–23 – Baghdad) and 6 male D. carinata , none of which bore even a faint line where the connecting endophallic sclerite would be found ( Fig. 20 View FIGURES 19–23 — Baghdad). The lack of intermediate forms in field collected material of 98 male of D. carinata and 69 male of D. sublineata , is evidence of reproductive isolation. Neither males nor females of D. sublineata and D. carinata were more difficult to identify to species near the contact zone of Iraq as would be expected were these to be interbreeding subspecies. Previously discussed differing component ratios in the putative aggregation pheromones of D. carinata and D. sublineata and the contrasting results of crossing each of these species with D. elongata are additional evidence of reproductive isolation.
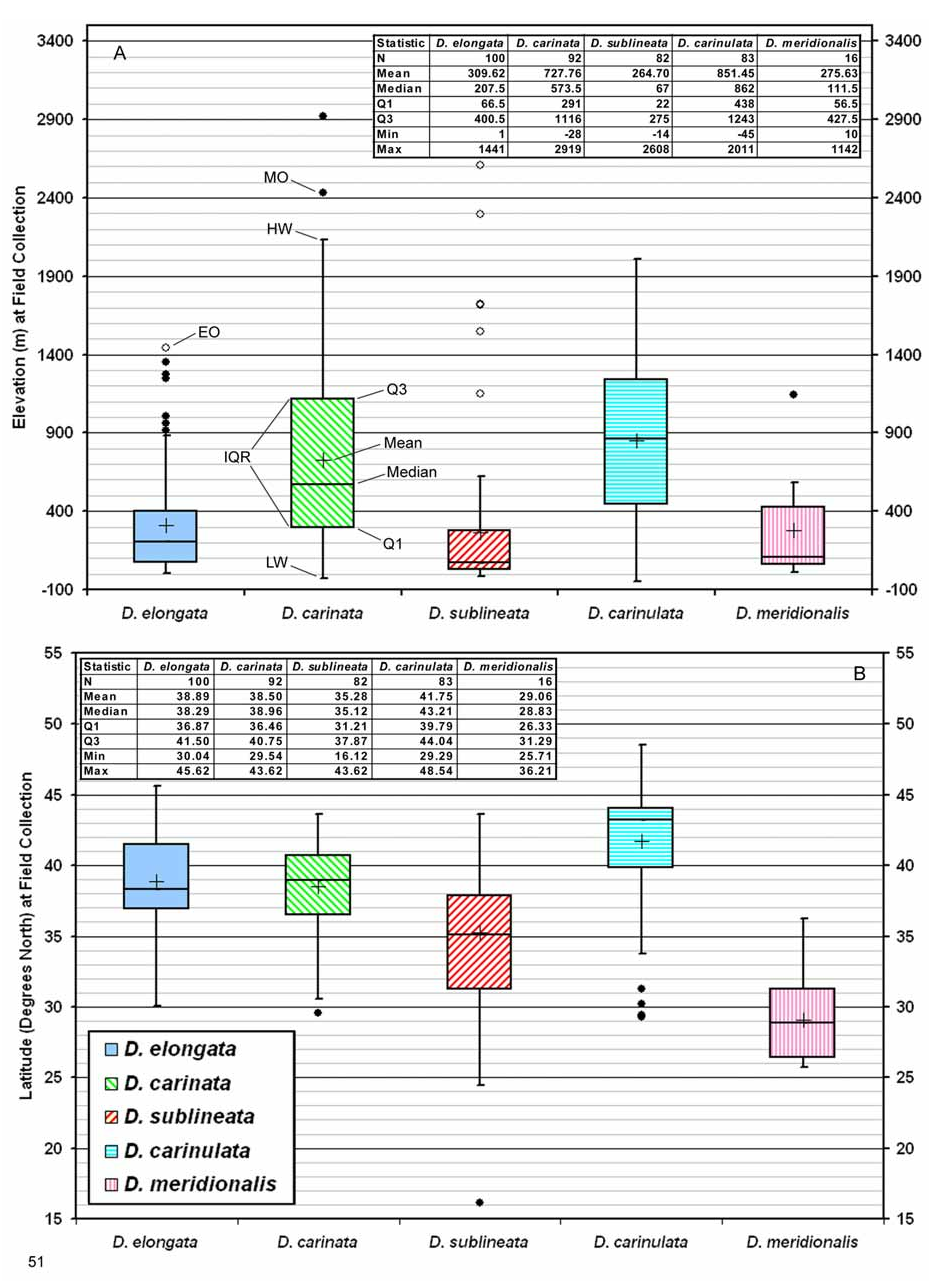
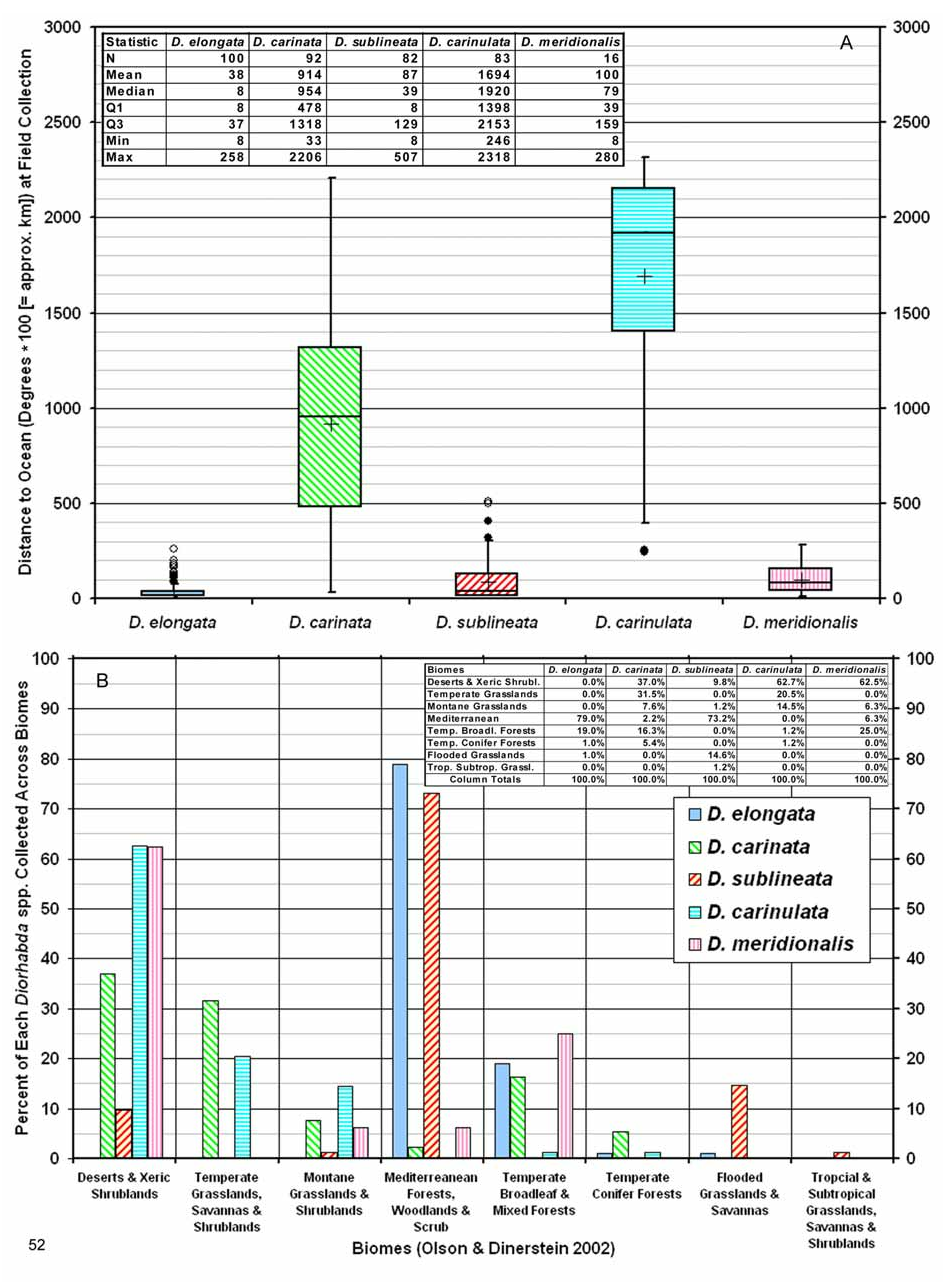
Common Name. The vernacular name “subtropical tamarisk beetle” refers to most of the main distribution of D. sublineata (31– 38°N; Fig. 51B View FIGURE 51 ) falling within the subtropical climates of North Africa (region from ca. 23.5– 35°N with mild winters). This subtropical distribution contrasts with that of the marginally sympatric sibling species, D. elongata , which has its main distribution (37– 41°N) entirely north of 35°N and which is rarely found below 35°N ( Fig. 51B View FIGURE 51 ). Diorhabda sublineata has the strongest presence of any Diorhabda sibling species in subtropical to tropical biomes, including the Flooded Grasslands and Savannas and the Tropical and Subtropical Grasslands, Savannas and Shrublands ( Table 9; Fig. 52B View FIGURE 52 ).
Biology. Host Plants. Diorhabda sublineata (as D. elongata ) is reported from T. africana Poiret and T. boveana Bunge (as T. bounopaea Gay ) in Algeria ( Peyerimhoff 1926) and T. africana and Tamarix sp. in Morocco ( Jolivet 1967) ( Table 1). Reports of T. gallica as a host of D. elongata in France ( Laboissière 1934) and Spain (Huelva Province, Hopkins and Carruth 1954) should also refer to D. sublineata . Tamarix senegalensis de Candolle is a new host record from collections in Ndiol Nar, Senegal by P. Jolivet (BMNH collection). Tamarix aphylla is a new host for D. sublineata (as D. elongata ) derived from a single adult collected by A. Kirk (pers. comm.) 20.2 km north of Douz, Tunisia. We examined other specimens of D. sublineata that Kirk collected from Tamarix sp. (not T. aphylla ) at six locations in Tunisia. Diorhabda sublineata is reported from Tamarix spp. in Egypt ( Boehm 1908, Alfieri 1976) and has been found in several locations along the Nile River where T. mannifera (Ehrenberg) Bunge and T. nilotica (Ehrenberg) Bunge , close relatives of T. senegalensis ( Baum 1978) are common, and these species may also serve as hosts. The center of distribution of T. canariensis is in Algeria, Morocco and Spain ( Baum 1978), and it is another likely host (Map 4). Tamarix arabica Bunge occurs in Yemen ( Baum 1978), where it could serve as a host of D. sublineata .
No-choice larval host suitability studies by Milbrath and DeLoach (2006a) confirm that D. sublineata larvae from Tunisia can survive to adulthood only on plants of the order Tamaricales , including Tamarix (Tamaricaceae) and, to a significantly lesser degree, on three North American Frankenia spp. (Frankeniaceae) : F. salina , F. johnstonii , and F. jamesii . In field cage no-choice studies, oviposition by D. sublineata on F. jamesii and F. johnstonii was not different from that on non-host coyote willow ( Salix exigua Nutall ) and adults experienced increased mortality compared to T. ramosissima × T. chinensis treatments (Milbrath and DeLoach 2006a). Multiple-choice adult oviposition studies in field cages (Milbrath and DeLoach 2006a) revealed that the three North American Frankenia spp. provide a little attraction for oviposition compared to Tamarix . However, surveys by Alan Kirk in Tunisia of May 2000 revealed D. sublineata (as D. elongata ) on Tamarix spp. from five of eight collection sites, but no Diorhabda were found on Frankenia at six collection sites in areas near Tamarix , even where Frankenia was found adjacent to Tamarix on which D. sublineata was abundant ( DeLoach et al. 2003b; A. Kirk, pers. comm.). In field cage multiple-choice studies, D. sublineata oviposited significantly less on T. aphylla than on most of the invasive North American tamarisks, including T. ramosissima , T. ramosissima × T. chinensis , T. ramosissima × T. canariensis / T. gallica , T. chinensis × T. canariensis / T. gallica , and T. parviflora (Milbrath and DeLoach 2006a, Milbrath and DeLoach 2006b). Oviposition did not differ between T. aphylla and T. canariensis / T. gallica in one of these field cage tests (Milbrath and DeLoach 2006b) while oviposition was significantly lower on T. aphylla than on T. canariensis / T. gallica in the other test (Milbrath and DeLoach 2006a). For oviposition, T. aphylla is accepted by D. sublineata to the same degree as T. ramosissima × T. chinensis in nochoice field cage studies (Milbrath and DeLoach 2006b). Tamarix aphylla is at moderate risk to damage by D. sublineata in the field and the degree to which D. sublineata would damage T. aphylla is difficult to predict, especially in the absence of other Tamarix spp. (Milbrath and DeLoach 2006b). Moran et al. (in press) found that D. sublineata (ex: Sfax, Tunisia) × D. elongata (ex: Sfakaki, Crete, Greece) hybrids demonstrate a clear preference to T. canariensis / T. gallica over T. aphylla in open-field tests near Kingsville, Texas. Frankenia is at very low risk of damage from D. sublineata (Milbrath and DeLoach 2006a) . Risk of damage to both T. aphylla and Frankenia by D. sublineata is probably much lower when these plants are not in the proximity of preferred Tamarix spp. (e.g., Blossey et al. 2001).
Ecology and Phenology. We found no reports on the ecology and phenology of D. sublineata in the Palearctic. Adult collection dates in the literature and our examined material are from January to November in Egypt, 22 January to 14 December in Tunisia (both from examined material), January to September in Algeria ( Peyerimhoff 1926, Doguet, pers. comm.) and April to October in France and Spain (this study). From these data, adults are found almost year round in North Africa.
Milbrath et al. (2007) found that D. sublineata (as D. elongata from Sfax, Tunisia) overwintering at Temple, Texas had ca. 92% survival from early November through the beginning of March but survival dropped to ca. 62–67% by mid-March when the tamarisk leaves were just budding. Overwintered adults began ovipositing in late March giving rise to five generations and a partial sixth generation. Fifth generation adults emerging in early September oviposited for several weeks before ceasing oviposition in November when they appeared to enter diapause.
Development and Reproduction. Milbrath et al. (2007) found that, at 28°C, D. sublineata (as D. elongata from Sfax, Tunisia) had a development time of 18.6 days from egg to adult (with 89% survival), a fecundity of 208 eggs, and a population doubling time of 5.5 days. These values were all very similar to those found for D. carinulata (Turpan and Fukang) , D. elongata (Crete) , and D. carinata ( Uzbekistan) (all as D. elongata ) in the same study.
Natural Enemies. Nosema sp. microsporidians infected D. sublineata larvae and adults collected southeast of Marith, Tunisia in 2008 (shipment EBCLGR-JK-2008-004) (D. Bean, pers. comm.).
Biogeography. Comparative. Diorhabda sublineata differs from other tamarisk beetles by the following combination of biogeographic characteristics: (1) strongly maritime and generally found within ca. 200 km from a sea coast, usually below 300 m elevation (but can range to 2,600 m elevation); (2) favors subtropical Mediterranean woodlands or Flooded Grasslands, Savannas and Shrublands biomes; and (3) latitudinal range of 16– 44°N and most commonly collected from 31– 38°N ( Table 7; Figs. 51–52 View FIGURE 51 View FIGURE 52 ). In contrast, the marginally sympatric D. elongata has a southern range limited to 30°N and it is much less common south of 35°N. In addition, D. elongata is rare or lacking in flooded grassland and desert biomes in which D. sublineata is found. Although both D. elongata and D. sublineata share T. gallica as one of their hosts ( Table 1), we have seen no mixtures of D. sublineata and D. elongata within series collected from specific localities such as are found with D. carinata and D. carinulata , or D. carinata and D. meridionalis . This may be the result of D. sublineata dominating in areas where it is marginally sympatric with D. elongata in Spain, Algeria, Egypt and probably elsewhere along the Mediterranean coast of North Africa (Map 1, Table 8; see also discussion under Biogeography for D. elongata ). The possibility that D. sublineata may occur in Italy where it may be rare and dominated by D. elongata should be investigated.
Diorhabda sublineata is marginally sympatric with D. carinata in Baghdad, Iraq, where their ranges meet, and it is allopatric with D. carinulata (Map1; Table 8). Both D. carinata and D. carinulata differ from D. sublineata in being primarily continental in distribution, mostly found in desert and grassland biomes rather than the Mediterranean biome, and being common north of 38°N ( Figs. 51–52 View FIGURE 51 View FIGURE 52 ). Diorhabda sublineata is also allopatric with D. meridionalis (Map 1; Table 8). Diorhabda meridionalis differs from D. sublineata in being more commonly collected further south at 26– 30°N, and in preferring the desert biome over the Mediterranean biome ( Figs. 51–52 View FIGURE 51 View FIGURE 52 ).
Descriptive. Diorhabda sublineata primarily inhabits the southwestern Palearctic realm and is the only member of the D. elongata group known to occur in the Afrotropical biogeographic realm (in Senegal and Yemen) (Map 1). It has most commonly been collected from the Mediterranean Forests, Woodlands and Scrub biome from 31– 38°N ( Fig. 52B View FIGURE 52 ). The next most important biome for D. sublineata is the Flooded Grasslands and Savannas biome from ca. 24– 34°N (Map 4; Fig. 42B View FIGURES 39–43 ). Diorhabda sublineata is also found in two additional biomes: the Deserts and Xeric Shrublands biome from 25– 33°N in North Africa and Iraq (at ca. 20–600 m elevation), and the Montane Grasslands and Shrublands biome to ca. 2,600 m elevation at Tacheddirt, Morocco (latitude 31°N) (Map 4; Table 7).
Diorhabda sublineata occurs primarily in three ecoregions of the Mediterranean Forests, Woodlands and Scrub biome (Olson and Dinerstein 2002), based on frequency of collections (Map 4): Mediterranean Woodlands and Forests of coastal Morocco, Algeria and Tunisia (at ca. 0 to 1,700 m), Mediterranean Acacia- Argania Dry Woodlands and Succulent Thickets of Morocco (at ca. 0 to 600 m), and Mediterranean Dry Woodlands and Steppe of Algeria and Tunisia (at ca. 0 to 600 m). Primary ecoregions of D. sublineata in the Flooded Grassland and Savanna biome are the Nile Delta Flooded Savanna of Egypt (at ca. 0 to 80 m elevation) and Saharan Halophytics of Algeria and Tunisia (at ca. 20 to 120 m elevation). Diorhabda sublineata was the most common galerucine reported in Egypt in 1908 ( Boehm 1908).
Diorhabda sublineata is found only in the Mediterranean Forests, Woodlands and Scrub biome in the northern portion of its range at ca. 37– 42°N in France and Spain (Map 4). Diorhabda sublineata (as D. elongata ) is common but not damaging in parts of southern Spain (Hopkins and Carruth 1954) and it is uncommon along Mediterranean coast of northeastern Spain ( Petitpierre 1988) and southern France ( Laboissière 1934, Chatenet 2002).
We found few reports of D. sublineata in the southern portion of its range from 16– 25°N in Africa and the Arabian Peninsula (Map 4). Additional collection efforts may reveal it to be more common in these areas. P. Jolivet (pers. comm.) reported August populations in Senegal reaching the thousands where he collected material that we examined from the Tropical and Subtropical Grasslands, Savannas and Shrublands biome.
The northwestern portion of the range of D. sublineata ( France, Spain, Morocco, Algeria and Tunisia) closely follows that of known host T. boveana and the suspected host T. canariensis (Map 4). Both hosts T. gallica and T. africana widely overlap the northwestern range of D. sublineata , but extend further east into Italy from which D. sublineata is not known. In Egypt, the distribution of T. nilotica and T. mannifera along the Nile River ( Baum 1978) corresponds well with that of D. sublineata .
Potential in Tamarisk Biological Control. Summary. The subtropical tamarisk beetle can potentially be effective in biological control of T. ramosissima / T. chinensis and hybrids with T. canariensis / T. gallica in the extreme southwestern U.S. Diorhabda sublineata may be the most suitable Diorhabda species for introduction into the Mediterranean biome below 37°N in California and the southern Chihuahuan Desert in Mexico (Map 13) (including the Mapimian and Saladan regions of Map 6 in Morafka [1977]). In the absence of D. meridionalis , D. sublineata is probably the most suitable species for a major portion of maritime subtropical deserts such as the Sonoran Desert and Tamaulipan Mezquital xeric shrubland.
Discussion. We find no anecdotal reports of the subtropical tamarisk beetle damaging Tamarix in the Old World, but this species can be locally abundant in places such as Senegal and Kom Ombo, Egypt, from which a large series of 106 specimens was collected in November. Tamarix ramosissima and its relatives are not recorded hosts of D. sublineata . Hybrids of T. ramosissima / T. chinensis with T. canariensis / T. gallica are common in Texas (J. Gaskin, pers. comm.), and D. sublineata may prefer these hybrids more than would other Diorhabda . Diorhabda sublineata has a moderate risk of damaging T. aphylla (Milbrath and DeLoach 2006b) and very low risk of damaging Frankenia (Milbrath and DeLoach 2006a) , and both these risks are probably much reduced at less proximity to preferred Tamarix spp. (e.g., Blossey et al. 2001).
The subtropical tamarisk beetle is most commonly found in the Mediterranean Forests, Woodlands and Scrub biome at 31– 38°N (Map 4, see Biogeography). In North America, Mediterranean ecoregions corresponding to this area include the California Coastal Sage and Chaparral ecoregion of California and Baja California, the California Montane Chaparral and Woodlands and southern portions of the California Interior Chaparral and Woodlands (Map 13). Additional North America habitats should be similar to where D. sublineata is found in the subtropical maritime Flooded Grassland and Savannah biome from 24– 34°N in North Africa that are surrounded by the subtropical maritime Deserts and Xeric Shrublands biome. In North America, this habitat particularly includes lower elevation (below 300m) portions of maritime subtropical Deserts and Xeric Shrublands biome from ca. 24– 35°N in the following ecoregions: southern Mojave Desert, Sonoran Desert, Tamaulipan Mezquital along the lower Rio Grande, and Tamaulipan Mattoral (Map 13). Along the Rio Grande near Presidio in the Big Bend region, the distribution of the Rio Grande cottonwood ( P. deltoides subsp. wislizenii ) more common in the Trans-Pecos Chihuahuan transitions to the more southern Chihuahuan desert species, Meseta cottonwood ( Populus fremontii subsp. mesetae ) ( Powell 1998). Correspondingly, the Big Bend area near Presidio may correspond with a transition zone for more northern desert D. carinata and D. carinulata into more southern desert D. sublineata . Morafka (Map 6 of Morafka 1977) mapped a transition from northern Trans-Pecos Chihuahuan Desert to more southern Mapimian Chihuahuan Desert at approximately the interface of our predicted ranges of D. carinata / D. carinulata and D. sublineata in the Big Bend region (Map 13). Tamarix aphylla is not a primary host for D. sublineata , but it is an indicator of the subtropical climate in North Africa where D. sublineata is most common (Map 4), and the most suitable areas in North America for D. sublineata may also occur within the area warm enough for T. aphylla to flourish (see Map 7). In the absence of D. meridionalis , D. sublineata would be the most suitable species for all of the Tamaulipan Mezquital in Texas according to our HSI model. Diorhabda sublineata appears to be well adapted to more southern latitudes in terms of initiation of diapause at shorter photoperiods (later in season) in the south (Bean and Keller in prep.) and can have high overwintering survival (ca. 65%) at Temple ( Milbrath et al. 2007). Species distribution models incorporating climatic data are in preparation to better predict habitat suitability.
Efforts to establish populations of D. sublineata from near Sfax, Tunisia near Ricardo, Texas on T. canariensis / T. gallica were not successful, possibly partly due to the small isolated poorly vigorous stand of tamarisk at this site. Plans are being made to release D. sublineata from near Marith, Tunisia in 2009 at better quality release sites with larger and more vigorous T. chinensis / T. canariensis stands along the Rio Grande and tributary streams in west and south Texas, such as at Ruidosa. Releases of any Diorhabda in southern California are awaiting clearance with USDA-APHIS (T. Dudley, pers comm.).
| NMPC |
National Museum Prague |
| ZMAN |
Instituut voor Taxonomische Zoologie, Zoologisch Museum |
| HNHM |
Hungarian Natural History Museum (Termeszettudomanyi Muzeum) |
| NHMB |
Natural History Museum Bucharest |
| DEI |
Senckenberg Deutsches Entomologisches Institut |
| ZIN |
Russian Academy of Sciences, Zoological Institute, Zoological Museum |
| USNM |
Smithsonian Institution, National Museum of Natural History |
| MZLU |
Lund University |
| SL |
University of Sierra Leone, Njala University College |
| PES |
University of Peshawar |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Diorhabda sublineata ( Lucas, 1849 ) REVISED STATUS
| Tracy, James L. & Robbins, Thomas O. 2009 |
Diorhabda elongata
| Wilcox, J. A. 1971: 63 |
| Normand, H. 1937: 126 |
Diorhabda elongata
| Laboissiere, V. 1934: 54 |
Diorhabda elongata
| Warchalowski, A. 2003: 328 |
| Alfieri, A. 1976: 233 |
| Normand, H. 1937: 126 |
| Laboissiere, V. 1934: 53 |
| Weise, J. 1924: 78 |
Diorhabda sublineata:
| Boehm, R. 1908: 68 |
Diorhabda elongata var. sublineata:
| Weise, J. 1893: 1132 |
Diorhabda elongata:
| Dudley, T. L. & Dalin, P. & Bean, D. W. 2006: 137 |
| Dudley, T. L. 2005: 13 |
| Dudley, T. L. 2005: 42 |
| Lopatin, I. K. & Aleksandrovich, O. R. & Konstantinov, A. S. 2004: 127 |
| DeLoach, C. J. & Carruthers, R. I. & Rodriguez-del & Bosque, L. A. 2003: 230 |
| Milbrath, L. R. & Herr, J. C. & Knutson, A. E. & Tracy, J. L. & Bean, D. W. & Rodriguez-del-Bosque, L. A. & Carruthers, R. I. & DeLoach, C. J. 2003: 225 |
| Warchalowski, A. 2003: 328 |
| Chatenet, G. du 2002: 223 |
| Anonymous 2001: 52 |
| Campobasso, G. & Colonnelli, E. & Knutson, L. & Terragitti, G. & Cristofaro, M. 1999: 145 |
| Kovalev, O. V. 1995: 78 |
| Petitpierre, E. 1988: 93 |
| Jolivet, P. 1967: 331 |
| Torres Sala, J. de 1962: 327 |
| Kocher, L. 1958: 109 |
| Normand, H. 1937: 126 |
| Ogloblin, D. A. 1936: 79 |
| Laboissiere, V. 1934: 54 |
| Peyerimhoff 1926: 359 |
| Correa de Barros, J. M. 1924: 9 |
| Weise, J. 1893: 635 |
| Bedel, L. 1892: 158 |
| Heyden, L. V. & Reitter, E. & Weise, J. 1891: 375 |
Galeruca elongata
| Joannis, M. L. 1866: 83 |
Galeruca sublineata
| Lucas, P. H. 1849: 542 |