ODONATA, Several D Tillyard, 1914
|
publication ID |
https://doi.org/10.5531/sd.sp.55 |
|
DOI |
https://doi.org/10.5281/zenodo.7733218 |
|
persistent identifier |
https://treatment.plazi.org/id/038D8781-FFCA-2077-FEE8-FA64A431FBDB |
|
treatment provided by |
Felipe |
|
scientific name |
ODONATA |
| status |
|
ORDER ODONATA View in CoL
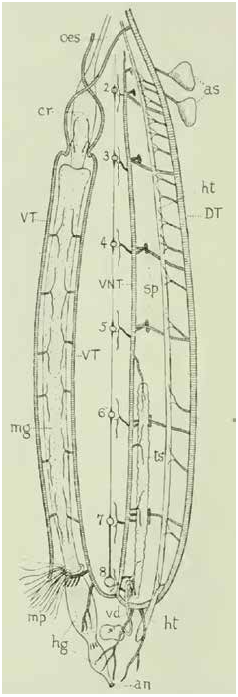
Respiration in Odonata , especially aquatic naiad immatures, has been the subject of investigation for some time, although much of the morphological work was performed more than a century ago. Scott (1905) presented one of the earliest detailed studies on dragonfly tracheae using a libellulid immature, highlighting notable characteristics such as the crossover of longitudinal trunks leading from the thorax to the abdomen, a trait retained in the adult. Tillyard (1917) remains the standard for dragonfly morphology, and he carefully diagrammed the tracheal architecture via dissection, although also with an emphasis on immatures. Chapman (1918) included both Anax and Lestes in his comparative study of leg-wing tracheal morphology. Kennedy (1922) built on Chapman’s work, extending it to abdominal tracheae; his extension to multiple orders insects using only odonate specimens is shown here to be insufficient. Recent studies have investigated physiological aspects, including active tracheal compression (likely related to respiration) in Odonata observed through synchrotron imaging by Westneat et al. (2003). For this study, three odonates were scanned, an aeshnid dragonfly larva at 30 µm resolution, an adult aeshnid at 21 µm resolution, and a calopterygid damselfly at 19 µm. As noted above, the wings of the adult aeshnid were removed and scanned separately; wing scans are not included here, though wing-base tracheae are retained and labeled.
Tillyard (1917) identified three pairs of longitudinal trunks in his figure 75 (included here as fig. 28 View FIGURE 28 ), whereas the aeshnid and calopterygid specimens here possess four, a condition not seen in any other order. A n -DLT is comprised of elongated and compartmentalized air-saclike tracheae connected segmentally or perhaps intermittently by regular tubular tracheae, and these were likely misidentified by Tillyard as air sacs rather than tracheae, which were discernible as such with micro-CT.
Scott (1905) indicated several tracheae present in the mid and hind legs of immatures but absent in adults, also noted by Chapman (1918), although with his own terminology. It is possible that these extra tracheae develop into the air-sac tracheae, which seem to be largely absent overall in immatures (otherwise they would have difficulty swimming underwater). Future studies should investigate this proliferation of tracheae and their possible development into the numerous air-sac tracheae.
The thorax of both specimens features a large number of air spaces. The flight musculature of odonates is thought to grow substantially during maturation of the adult ( Marden, 1989; Anholt et al., 1991; Marden, 2000). It could be that these air spaces allow expansion of flight muscle—as the adult exoskeleton is fixed in size, empty volume must be allocated in advance to allow for an increase in flight muscle mass. Interestingly, this would be the inverse of the “gas bag in the gut” phenomenon (see Discussion), where the alimentary canal is coopted into a large air space in the adult.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |