Namalycastis caetensis, Alves, Paulo Ricardo & Santos, Cinthya Simone Gomes, 2016
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4144.4.3 |
|
publication LSID |
lsid:zoobank.org:pub:0D30B35D-4F0C-4C14-BC66-0B8A433DF6B3 |
|
DOI |
https://doi.org/10.5281/zenodo.5687657 |
|
persistent identifier |
https://treatment.plazi.org/id/038D87F9-BA25-FFF2-5C86-FA460987FC52 |
|
treatment provided by |
Plazi |
|
scientific name |
Namalycastis caetensis |
| status |
sp. nov. |
Namalycastis caetensis View in CoL sp. nov.
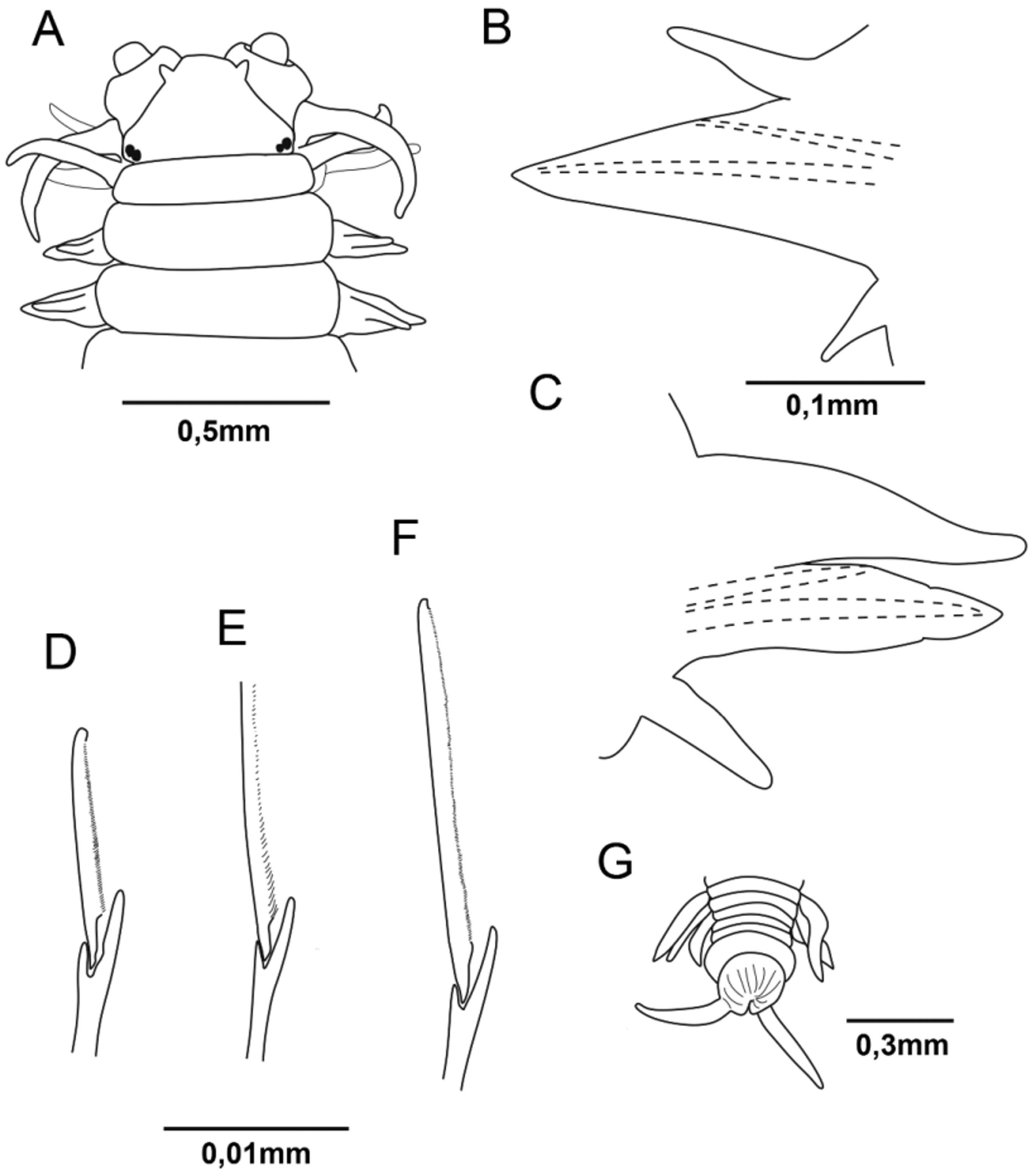
Figs 1 View FIGURE 1 A–G, 2 A–D.
Type material. Holotype ( MNRJ /P771), complete and well-preserved specimen, found in mangroves of the Caeté river estuary, Pará, Brazil (0°59’25.924”S, 46°44’12.101”W) in shallow waters with low salinity, June of 2005 GoogleMaps . Paratypes, six specimens, three collected in Caeté river ( MNRJ /P772, 1°3’5.640”S, 46°45’11.088”W), in muddy sediment under freshwater GoogleMaps , 1.5 m deep; other three collected in Guajará bay ( MNRJ /P773, 01°28’32.2”S, 48°25’46.3”W) also in freshwater GoogleMaps , 2 m deep.
Description. Holotype complete, 29 mm long with 121 chaetigers and 0.7 mm wide at chaetiger 10. Body long with dorsum convex and venter flat. Widest mid-anteriorly, tapering anteriorly and posteriorly. Color pale, slightly pink in 70% ethanol. Dark brown epidermal pigment on pygidium.
Prostomium trapezoidal lacking anterior cleft or longitudinal groove. Prostomium with a slight lateral indentation. Antennae short and subconical, lateral and aligned over mid-palps ( Figs 1 View FIGURE 1 A and 2A). Two pairs of eyes, with posterior pair slightly smaller, aligned transversally to the longitudinal axis of the prostomium ( Figs 1 View FIGURE 1 A and 2A).
Tentacular cirri with cirrophores slightly indistinct and cirrostyles smooth. Anterodorsal pair is the longest. Posterodorsal tentacular cirri extending posteriorly to chaetiger 2 ( Figs 1 View FIGURE 1 A and 2A).
Parapodia with subconical acicular neuropodial ligule. Dorsal cirri increasing slightly in length posteriorly; 0.8–1.0x length of parapodium of chaetiger 3, 1.2–1.6x at chaetiger 50 and 1.3–1.4x at chaetiger 100 ( Figs 1 View FIGURE 1 B–C). Ventral cirri increasing slightly in length posteriorly; length of ventral cirri from posterior podia 1.1–1.3x length of ventral cirri from anterior parapodia.
Notopodial sesquigomph spinigers from chaetigers 3–4. Supra-neuroacicular chaetae as sesquigomph spinigers in postacicular fascicles and heterogomph falcigers in preacicular fascicles. Sub-neuroacicular chaetae as heterogomph spinigers and heterogomph elongated falcigers in postacicular fascicles and heterogomph falcigers in preacicular fascicles. Supra-neuroacicular falcigers in chaetiger 10 finely serrated, with blades 5.5–7.3x longer than width of shaft head ( Fig. 1 View FIGURE 1 D). Sub-neuroacicular falcigers in chaetiger 10 with blades finely serrated, dorsalmost 4.7–6.3x longer than width of shaft head, ventral-most 4.0–6.3x longer than width of shaft head. Subneuroacicular spinigers in chaetiger 10 with blades finely serrated ( Fig. 1 View FIGURE 1 E). Sub-neuroacicular falcigers in posterior region with blades finely serrated. Sub-neuroacicular spinigers in posterior region having increasingly coarse serrations proximally. Presence of heterogomph elongated falcigers in sub-postacicular fascicle having blades finely serrated ( Figs 1 View FIGURE 1 F and 2B–C). Chaetae pale, aciculae black.
Pygidium button-shaped multi-incised with dorsoterminal anus. Anal cirri subconical, smooth, ventral and 1.3– 2x width of pygidium ( Figs 1 View FIGURE 1 G and 2D).
Variation. Some individuals show epidermal pigmentation on the dorsum of posterior region and a cleft in the anterior end of prostomium, associated with a narrow longitudinal groove extending to mid-posterior region of prostomium. Some individuals with supra-neuroacicular falcigers in chaetiger 10 with blades finely serrated only proximally. These variations were found in both populations studied.
Etymology. The species has been named after one of the estuaries where it was found, Rio Caeté.
Habitats. Specimens were found in the infralittoral of mangrove regions along the northern coast of Brazil, in depths between 1 and 2 meters, in muddy sediments of low salinity to freshwaters.
Remarks. Before discussing the new species described here, we would like to address our use of the term “elongated falcigers”. We agree with Conde-Vela (2013) that the term “pseudospiniger” might not be the most appropriate as it suggests these are spinigerous chaetae. Instead, they have characteristics of a falciger and not of a spiniger, like the falcate tip for example ( Fig. 2 View FIGURE 2 B). However, there are differences between the chaeta here identified as elongated falcigers and a typical falciger. Besides having different lengths, these two kinds of blades do not appear in the same fascicle of the neuropodia ( Fig. 2 View FIGURE 2 C). Usually a typical falciger is inserted in preacicular fascicles, while elongated falcigers are inserted in postacicular fascicles. Namalycastis occulta, Conde-Vela, 2013 , is the only species of the genus that shows a type D arrangement of chaetae sensu Glasby (1999), which means that the elongated falciger (pseudospinigers) appear in the preacicular fascicle; a type of arrangement usually present in Namanereis species. Since falcigers with elongated blades only appear in postacicular fascicles, we understand that it represents a significant diagnostic character. We dismissed the use of the term “pseudospiniger” and adopted “elongated falcigers” to discriminate them from the usual preacicular falcigers with shorter blades.
The prostomium of the holotype and most specimens lacks anterior cleft and longitudinal groove. However, it was possible to observe this feature only in some individuals and mainly under SEM. For this reason, we treat this character as morphological variation and scored as a polymorphism in phylogenetic analyses. We also noticed that the tentacular cirri of Namalycastis caetensis sp. nov. have a slightly indistinct cirrophore. It is possible to observe the presence of the cirrophore in the specimens examined, but it is not as clearly observable as in other species of the genus, such as Namalycastis macroplatis Glasby, 1999 ( Glasby, 1999; Fig. 25a), for example. On the other hand, it is possible to identify the structure, so it should not be described as indistinct. Another character variation that deserves discussion is the size of the dorsal cirri along the body. Glasby (1999) described the relative sizes of dorsal cirri in relation to the parapodia of the same segment to evaluate the development of this feature along the body; an approach that we adopted here. However, this approach may hide the influence of the size of the parapodial lobes. In Namalycastis caetensis sp. nov., and in other species of the genus, the length of the parapodial lobes in posterior chaetigers is usually shorter than the parapodia of anterior regions. This means that the relative size of the dorsal cirri to the length of parapodia may represent a dubious character, and it is impossible to know if the resulting value reflects an increase in the length of the cirri or a decrease in the length of the parapodial lobe.
According to Amaral et al. (2013), six species of the genus Namalycastis have been registered along the Brazilian coast. The highest number of records is for the globally distributed Namalycastis abiuma . In his revision, Glasby (1999) described an informal taxon of “species group”, acknowledging the wide variation for N. abiuma . Despite that the author could not find sufficient variation to support new species descriptions, he asserted that the cosmopolitan status of the species must be revised since N. abiuma could represent a case of a cryptic multi-species group. The variation recognized by Glasby for N. abiuma includes some of the characters described here for N. caetensis sp. nov., like the length of posterior dorsal cirri and the presence of elongated falcigers (=pseudospinigers) for example. Since the type locality of N. caetensis sp. nov. is inside the range of distribution of the N. abiuma species group, our new species may represent some of the specimens previously described within the N. abiuma species group. We have decided not to consider these features as components of group variation, instead erecting the new species. Namalycastis caetensis sp. nov. differs from N. abiuma by having a small body, prostomium without anterior cleft, tentacular cirri with cirrophores slightly indistinct, acicular neuropodial ligulae subconical and the presence of elongated falcigers in the postacicular fascicle, which also differs the new species from N. borealis , N. brevicornis , N. hawaiiensis and N. multiseta . Other species that resembles the new species but differs in some characters are: Namalycastis kartaboensis that shows distinct cirrophores in tentacular cirri and falcigers with smooth blades in all body, Namalycastis longicirris that shows the dorsum highly arched and dorsal cirri increasing greatly posteriorly. The presence of elongated falcigers in the postacicular fascicle has also been described for Namalycastis nicoleae Glasby, 1999 . This latter species presents other similarities to N. caetensis sp. nov. like having a small body (usually less than 130 chaetigers), similar size of tentacular cirri and similar lengths of dorsal cirri in posterior parapodia, but differs in many other characters, like epidermal pigment, eye angle, falciger serration and antennae size.
TABLE ³. Character matrix. Legenđs: -. inapplicable;?. unknown characters; &. polymorphism.
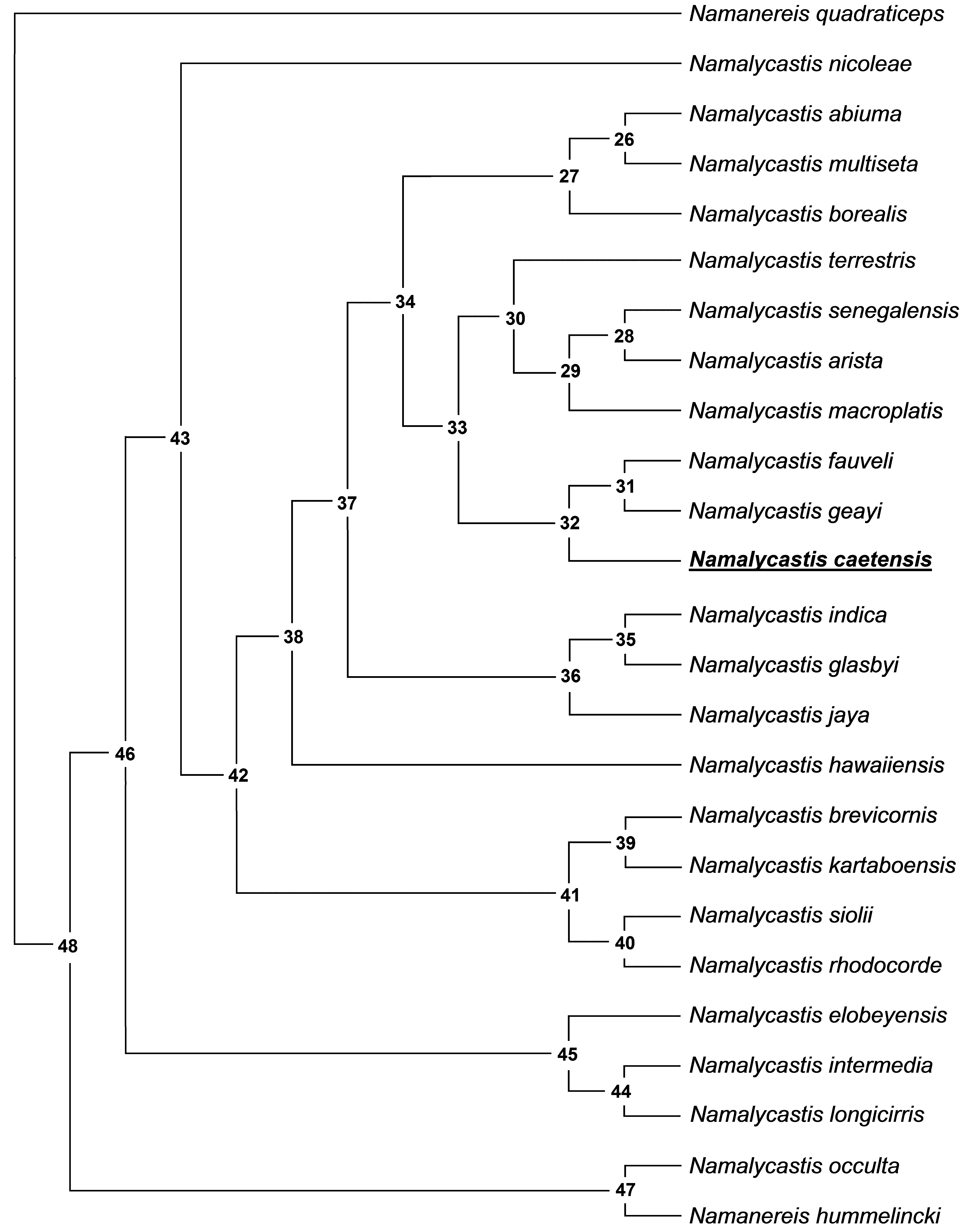
Taxa Characters Phylogenetic analyses. The heuristic search yielded 26 equally parsimonious trees with 122 steps. Trees retained had the following indices: CI=0.516, RI=0.553, RC=0.286 and HI=0.738. The second round of tree searching returned the same trees with the same indices as those of the first round, showing that all trees belonged to the same heuristic island. Character indices and an apomorphy list for one of the most parsimonious trees (chosen at random and represented in Figure 3 View FIGURE 3 ) are given in Table 4 View TABLE 4 and Table 5, respectively. Bootstrap support values for the Majority-rule consensus tree are shown in Figure 4 View FIGURE 4 .
The low support values obtained and the low consistency index for the trees indicates the close resemblance of species of the genus. This result was as expected since the group does not show many differentiating characters, making it difficult to identify informative phylogenetic features. Many diagnostic characters are apomorphic for terminal taxa, and these were not included in our analyses. We only show bootstrap support values above 50, since the bootstrap values obtained in our analyses were always low.
Here we described the tentacular cirrophore as being slightly indistinct. Glasby (1999) suggests that this character may be size-dependent, but we decided to include this character since there are some small species that show a clearly distinct tentacular cirrophore, like N. nicoleae Glasby, 1999 . We coded “slightly indistinct cirrophore” together with “indistinct cirrophore” because it is possible to identify this structure in some described species having indistinct cirrophores, like Namalycastis geayi ( Gravier, 1901) , ( Glasby, 1999 Fig. 18a). We believe that what Glasby (1999) identified as being indistinct in some species is the same structure that we describe here as being “slightly indistinct”.
As discussed above, N. occulta shows some characters associated with the genus Namanereis , and this view is supported by the position of that species in our trees. Conde-Vela (2013) recognized that this species resembles some Namanereis species and argued that he regarded it as Namalycastis because of a series of diagnostic characters, like the number of tentacular cirri and the shape of prostomium and antennae. Indeed, N. occulta shows some diagnostic characters for both of these Namanereidinae genera, suggesting that additional questions about the evolutionary history of the subfamily need to be addressed. In our results, N. occulta is closer to Namanereis species than it is from the genus Namalycastis showing that this species may belong to the former genus.
Branch Character Steps ci Change
node 48 --> Namanereis quadraticeps 11 1 0.250 0 --> 1 15 1 0.400 1 ==> 0 18 1 0.200 0 --> 1 20 1 0.571 1 --> 2 27 1 0.778 1 --> 0&1 node 48 --> node 46 4 1 0.571 0 --> 1 17 1 1.000 0 ==> 1 21 1 0.500 0 ==> 1 node 46 --> node 44 15 1 0.400 1 ==> 2 18 1 0.200 0 --> 1 node 44 --> Namalycastis nicoleae 24 1 0.500 0 ==> 1 node 44 --> node 43 1 1 0.500 0 ==> 1 22 1 0.167 0 --> 1 25 1 0.500 0 ==> 1 node 43 --> node 38 5 1 0.750 0 ==> 1 node 38 --> node 37 3 1 0.250 0 ==> 1 10 1 0.750 0 --> 1 node 37 --> node 34 9 1 0.900 1 ==> 0 26 1 1.000 0 ==> 1 node 34 --> node 30 10 1 0.750 1 --> 0 node 30 --> node 27 20 1 0.571 1 ==> 2 node 27 --> node 26 7 1 0.167 1 ==> 0 node 26 --> Namalycastis abiuma 9 1 0.900 0 --> 0& 1 13 1 0.286 0 ==> 2 16 1 0.429 0 ==> 1 27 1 0.778 1 --> 0&1 node 26 --> Namalycastis multiseta 9 1 0.900 0 --> 0& 1 10 1 0.750 0 --> 0& 1 11 1 0.250 0 ==> 1
node 27 --> Namalycastis borealis 9 1 0.900 0 --> 0& 1 10 1 0.750 0 --> 0& 1 22 1 0.167 1 ==> 0 26 1 1.000 1 --> 0& 1 27 1 0.778 1 --> 0&1 node 30 --> node 29 2 1 0.333 0 ==> 1 4 1 0.571 1 --> 0 6 1 0.5 0 0 1 ==> 0 14 1 0.200 1 ==> 0 ......continued on the next page Branch Character Steps ci Change
node 29 --> node 28 3 1 0.250 1 ==> 0 22 1 0.167 1 ==> 0 node 28 --> Namalycastis fauveli 11 1 0.250 0 ==> 1 16 1 0.429 0 --> 0& 1 26 1 1.000 1 --> 0& 1 27 2 0.778 1 ==> 0&2 node 28 --> Namalycastis geayi 4 1 0.571 0 --> 0&1 5 1 0.750 1 --> 0&1 6 1 0.500 0 --> 0&1 9 1 0.9 0 0 0 ==> 2 26 1 1.000 1 --> 0&1 node 29 --> Namalycastis caetensis 1 1 0.5 0 0 1 ==> 0 4 1 0.571 0 --> 0& 1 15 1 0.400 2 ==> 1 24 1 0.500 0 ==> 1 node 34 --> node 33 13 1 0.286 0 ==> 1 node 33 --> Namalycastis terrestris 5 1 0.750 1 --> 0&1 9 1 0.900 0 --> 0& 1 22 1 0.167 1 ==> 0 node 33 --> node 32 16 1 0.429 0 ==> 1 19 1 0.333 1 ==> 0 node 32 --> node 31 2 1 0.333 0 ==> 1 3 1 0.2 5 0 1 ==> 0 node 31 --> Namalycastis senegalensis 16 1 0.429 1 --> 0&1 node 32 --> Namalycastis macroplatis 10 1 0.750 1 --> 0& 1 26 1 1.000 1 --> 0&1 node 37 --> node 36 2 1 0.333 0 ==> 1 11 1 0.250 0 --> 1 16 1 0.429 0 ==> 1 27 1 0.778 1 ==> 2 node 36 --> node 35 13 1 0.286 0 ==> 2 25 1 0.500 1 ==> 0 node 35 --> Namalycastis indica 3 1 0.2 5 0 1 ==> 0 9 1 0.900 1 --> 1& 2 10 1 0.750 1 --> 0& 1 15 1 0.400 2 ==> 1 27 1 0.778 2 --> 1&2 node 35 --> Namalycastis glasbyi 7 1 0.1 6 7 1 ==> 0 14 1 0.200 1 ==> 0 22 1 0.167 1 ==> 0 node 36 --> Namalycastis jaya 6 1 0.5 0 0 1 ==> 0 18 1 0.200 1 ==> 0 ......continued on the next page Branch Character Steps ci Change
node 38 --> Namalycastis hawaiiensis 13 1 0.286 0 ==> 1 18 1 0.200 1 ==> 0 node 43 --> node 42 20 1 0.571 1 ==> 0 node 42 --> node 40 16 1 0.429 0 ==> 1 node 40 --> Namalycastis brevicornis 26 1 1.000 0 --> 0&1 node 40 --> node 39 13 1 0.286 0 ==> 2 node 39 --> Namalycastis kartaboensis 4 1 0.5 7 1 1 ==> 0 9 1 0.900 1 --> 0& 1 10 1 0.750 0 --> 0&1 node 39 --> Namalycastis longicirris 7 1 0.1 6 7 1 ==> 0 14 1 0.200 1 ==> 0 20 1 0.571 0 ==> 1 node 42 --> node 41 6 1 0.500 1 ==> 0 10 1 0.750 0 ==> 1 node 41 --> Namalycastis siolii 5 1 0.7 5 0 0 ==> 1 15 1 0.400 2 ==> 1 19 1 0.333 1 ==> 0 20 1 0.571 0 --> 0&1 node 41 --> Namalycastis rhodocorde 7 1 0.1 6 7 1 ==> 0 18 1 0.200 1 ==> 0 21 1 0.500 1 ==> 0 node 46 --> node 45 9 1 0.900 1 --> 0 13 1 0.286 0 ==> 2 14 1 0.200 1 ==> 0 node 45 --> Namalycastis elobeyensis 4 1 0.571 1 --> 0& 1 20 1 0.571 1 --> 1& 2 22 1 0.167 0 --> 1 node 45 --> Namalycastis intermedia 7 1 0.1 6 7 1 ==> 0 9 1 0.900 0 --> 0& 1 19 1 0.333 1 ==> 0 27 1 0.778 1 ==> 2 node 48 --> node 47 8 1 1.000 1 ==> 0 12 1 1.000 1 ==> 0 23 1 1.000 0 ==> 1 node 47 --> Namalycastis occulta 4 1 0.571 0 --> 1 7 1 0.1 6 7 1 ==> 0 13 1 0.286 0 ==> 1 16 1 0.429 0 ==> 1 20 1 0.571 1 --> 2 node 47 --> Namanereis hummelincki 14 1 0.200 1 ==> 0 27 1 0.778 1 --> 1&2 Our trees show some similarities with those presented by Glasby (1999), like N. abiuma being closely related to N. multiseta Glasby, 1999 ; the basal position of N. elobeyensis Glasby, 1999 , N. intermedia Glasby, 1999 and N. nicoleae ; and the grouping of N. macroplatis , N. senegalensis and N. arista Glasby, 1999 based on the absence of preacicular falcigers in posterior parapodia. The description of some new taxa since the Glasby (1999) publication may have influenced the position of some species which did not group together in previous studies; in particular, those closely related to N. caetensis sp. nov. Glasby (1999) included Namanereis species in his analysis, so that phylogeny used a different set of characters and terminal taxa to ours, so some differences were expected.
Our results suggest that Namalycastis caetensis sp. nov. is closely related to Namalycastis geayi and Namalycastis fauveli Nageswara Rao, 1981 , based on four characters: Body widest mid-anteriorly, smooth prostomial anterior end, very short antennae and a slightly indistinct tentacular cirrophore. All three species show some unique diagnostic characters; N. geayi is the only species having spinigerous chaetae in all fascicles, N. fauveli has heterogomph chaetae with prolonged bosses on shaft head, and N. caetensis sp. nov. exhibits elongated falcigers in the postacicular fascicle. The bootstrap value for this clade is not high, however, few branches showed bootstrap support values higher than 50. The Namalycastis genus represents a group with few informative characters and includes some species that are poorly described and have little taxonomic information, which includes Namalycastis longicirris and Namalycastis siolii , for example. Possibly the lack of information for some species may have influenced our phylogeny.
Our results point to the difficulty of studying the phylogeny of the group since there are few informative characters to assess relationships within the genera and species. Only with more in-depth taxonomic studies will be possible to reassess morphological relationships within the group. Whilst many species still obscure to science, our description of a new species enhances insights into this group and its evolutionary relationships.
TABLE 4. Character indices for the maximum parsimony tree in Figure 3. (ci) Consistency index; (ri) Retention index. (rc); Rescaled consistency index; (hi) Homoplasy index; (G-fit) Goloboff- fit index.
| Character | Tree steps | Max steps G-steps | ci | ri | rc | hi | G-fit |
|---|---|---|---|---|---|---|---|
| 1 | 1 | 2 7 | 0.500 | 0.833 | 0.417 | 0.500 | 0.750 |
| 2 | 1 | 3 8 | 0.333 | 0.714 | 0.238 | 0.667 | 0.600 |
| 3 | 1 | 4 8 | 0.250 | 0.571 | 0.143 | 0.750 | 0.500 |
| 4 | 4 | 7 7 | 0.571 | 0.000 | 0.000 | 0.857 | 0.333 |
| 5 | 3 | 4 10 | 0.750 | 0.857 | 0.643 | 0.750 | 0.500 |
| 6 | 2 | 4 6 | 0.500 | 0.500 | 0.250 | 0.750 | 0.500 |
| 7 | 1 | 6 7 | 0.167 | 0.167 | 0.028 | 0.833 | 0.375 |
| 8 | 1 | 1 2 | 1.000 | 1.000 | 1.000 | 0.000 | 1.000 |
| 9 | 9 | 10 14 | 0.900 | 0.800 | 0.720 | 0.800 | 0.273 |
| 10 | 6 | 8 12 | 0.750 | 0.667 | 0.500 | 0.875 | 0.300 |
| 11 | 1 | 4 4 | 0.250 | 0.000 | 0.000 | 0.750 | 0.500 |
| 12 | 1 | 1 2 | 1.000 | 1.000 | 1.000 | 0.000 | 1.000 |
| 13 | 2 | 7 13 | 0.286 | 0.545 | 0.156 | 0.714 | 0.375 |
| 14 | 1 | 5 8 | 0.200 | 0.429 | 0.086 | 0.800 | 0.429 |
| 15 | 2 | 5 8 | 0.400 | 0.500 | 0.200 | 0.600 | 0.500 |
| 16 | 3 | 7 12 | 0.429 | 0.556 | 0.238 | 0.857 | 0.333 |
| 17 | 1 | 1 3 | 1.000 | 1.000 | 1.000 | 0.000 | 1.000 |
| 18 | 1 | 5 7 | 0.200 | 0.333 | 0.067 | 0.800 | 0.429 |
| 19 | 1 | 3 5 | 0.333 | 0.500 | 0.167 | 0.667 | 0.600 |
| 20 | 4 | 7 10 | 0.571 | 0.500 | 0.286 | 0.714 | 0.375 |
| 21 | 1 | 2 4 | 0.500 | 0.667 | 0.333 | 0.500 | 0.750 |
| 22 | 1 | 6 7 | 0.167 | 0.167 | 0.028 | 0.833 | 0.375 |
| 23 | 1 | 1 2 | 1.000 | 1.000 | 1.000 | 0.000 | 1.000 |
| 24 | 1 | 2 2 | 0.500 | 0.000 | 0.000 | 0.500 | 0.750 |
| 25 | 1 | 2 8 | 0.500 | 0.857 | 0.429 | 0.500 | 0.750 |
| 26 | 6 | 6 10 | 1.000 | 1.000 | 1.000 | 0.833 | 0.375 |
| 27 | 7 | 9 10 | 0.778 | 0.333 | 0.259 | 0.778 | 0.300 |
| MNRJ |
Museu Nacional/Universidade Federal de Rio de Janeiro |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |