Ectaesthesiidae, Ng & Ahyong & Castro, 2023
|
publication ID |
https://doi.org/ 10.26107/RBZ-2023-0047 |
|
publication LSID |
lsid:zoobank.org:pub:821BC4EC-5AF9-4727-84A3-C44839DFBE28 |
|
persistent identifier |
https://treatment.plazi.org/id/9E54A869-6F84-4D78-AAF4-F3DE23E42A43 |
|
taxon LSID |
lsid:zoobank.org:act:9E54A869-6F84-4D78-AAF4-F3DE23E42A43 |
|
treatment provided by |
Felipe |
|
scientific name |
Ectaesthesiidae |
| status |
new family |
Ectaesthesiidae View in CoL , new family
Type genus. Ectaesthesius Rathbun, 1898 View in CoL , by monotypy.
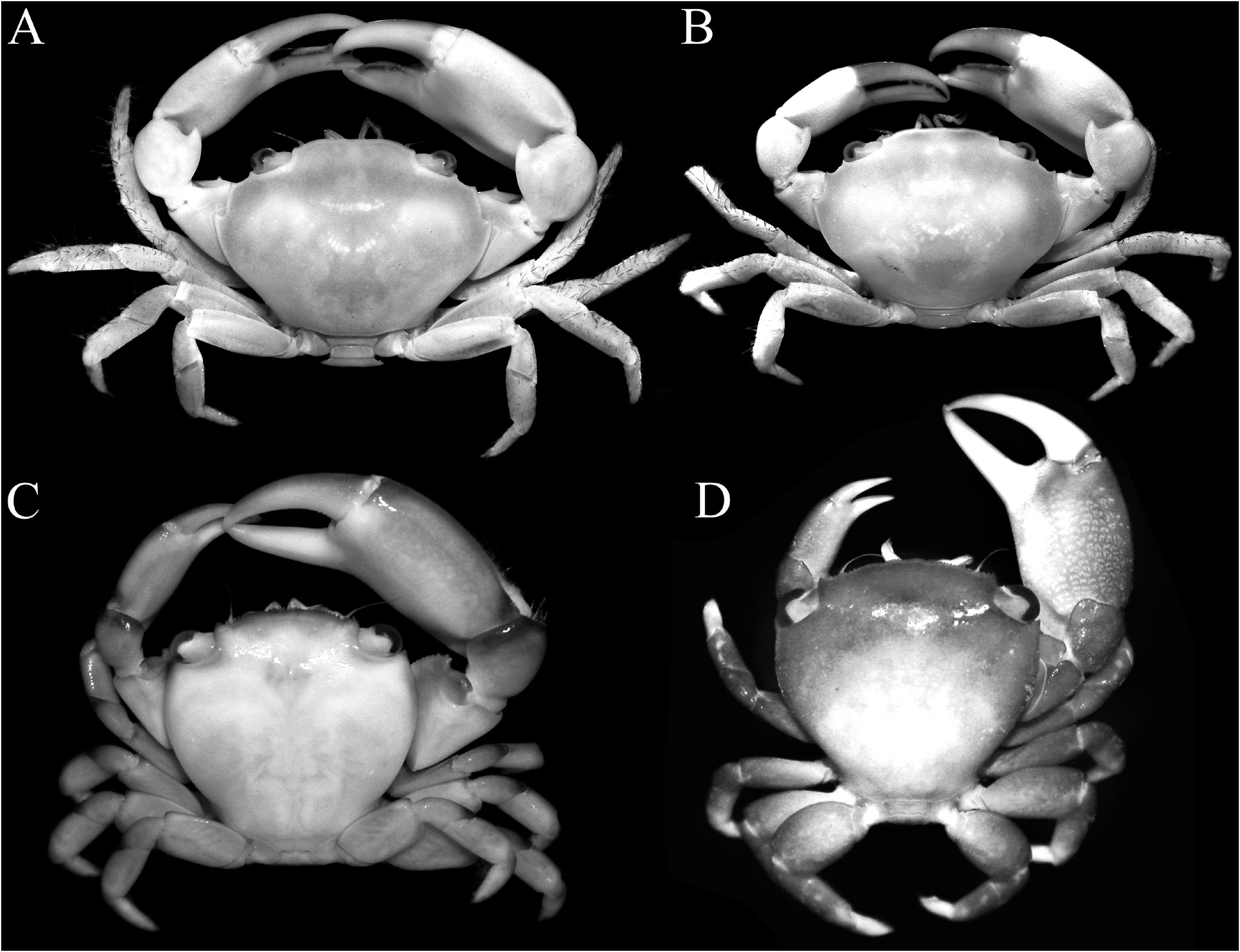
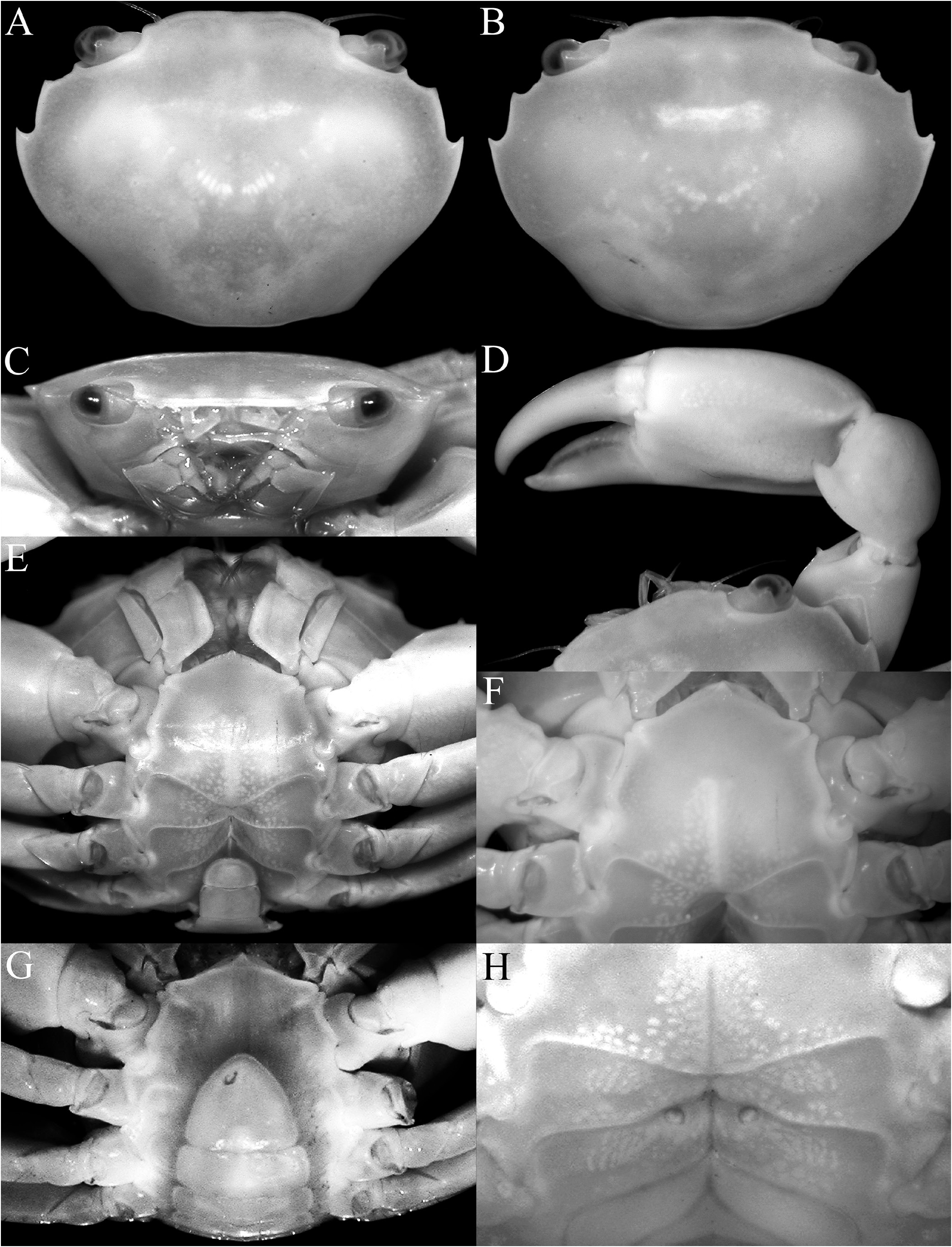
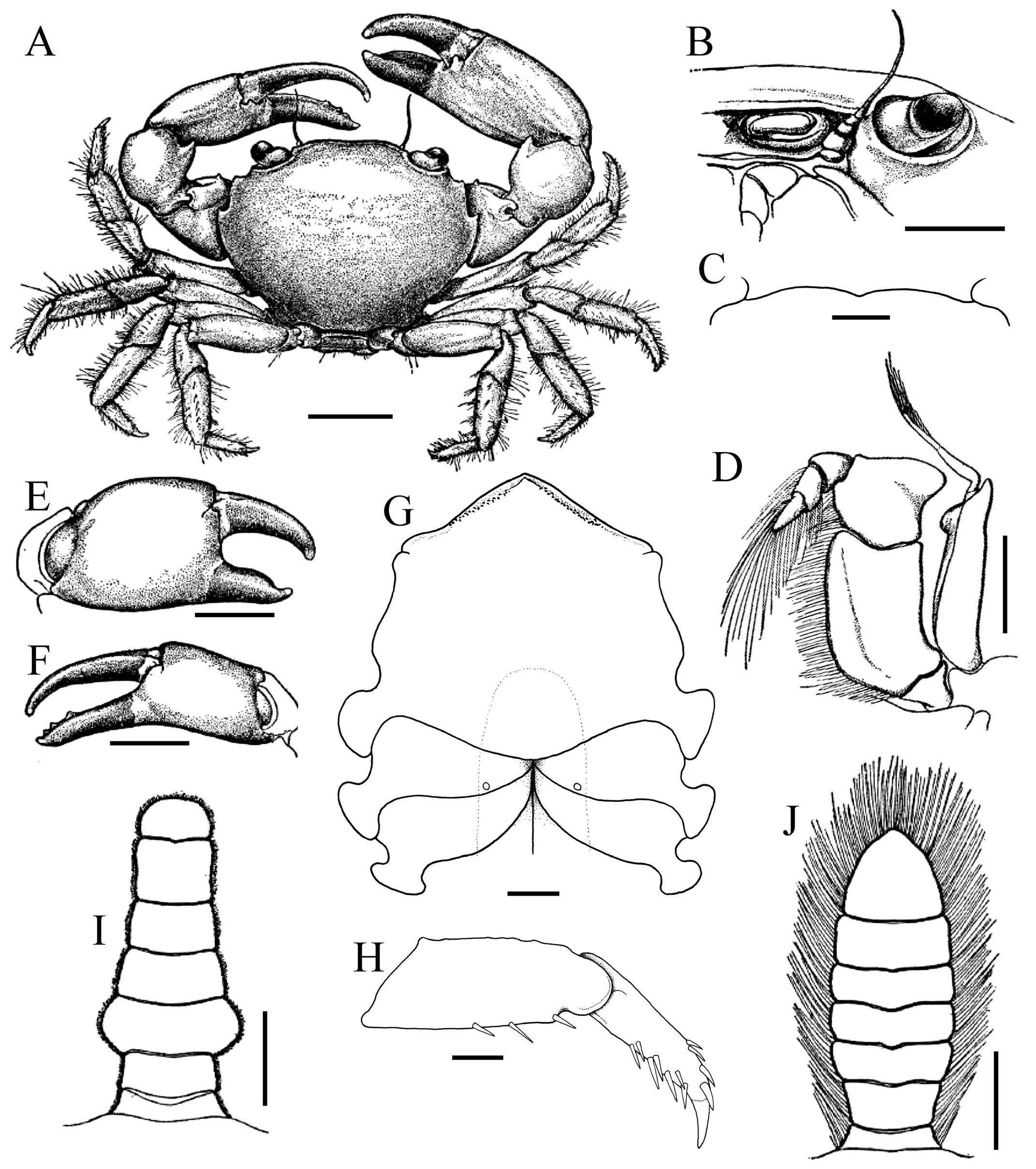
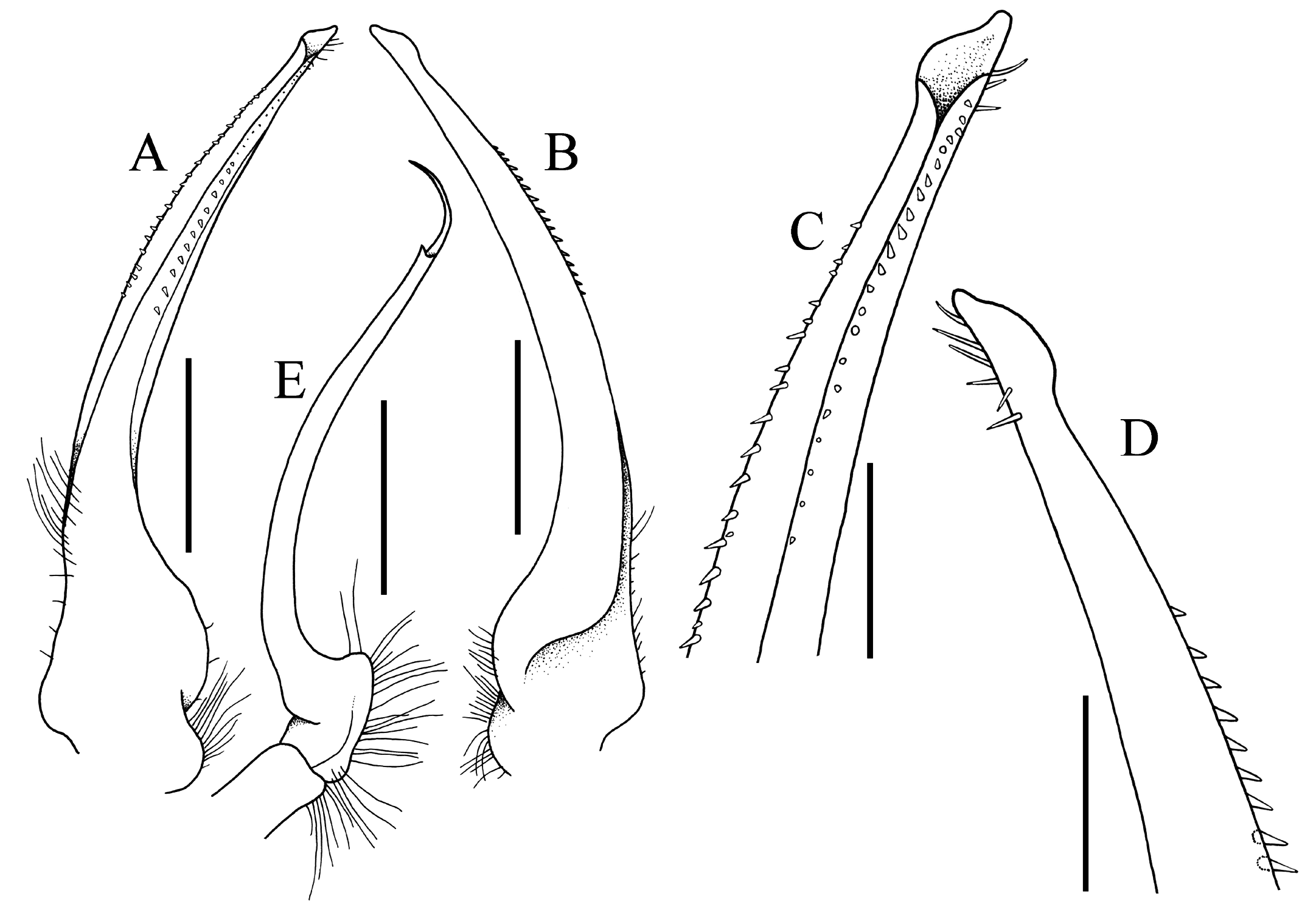
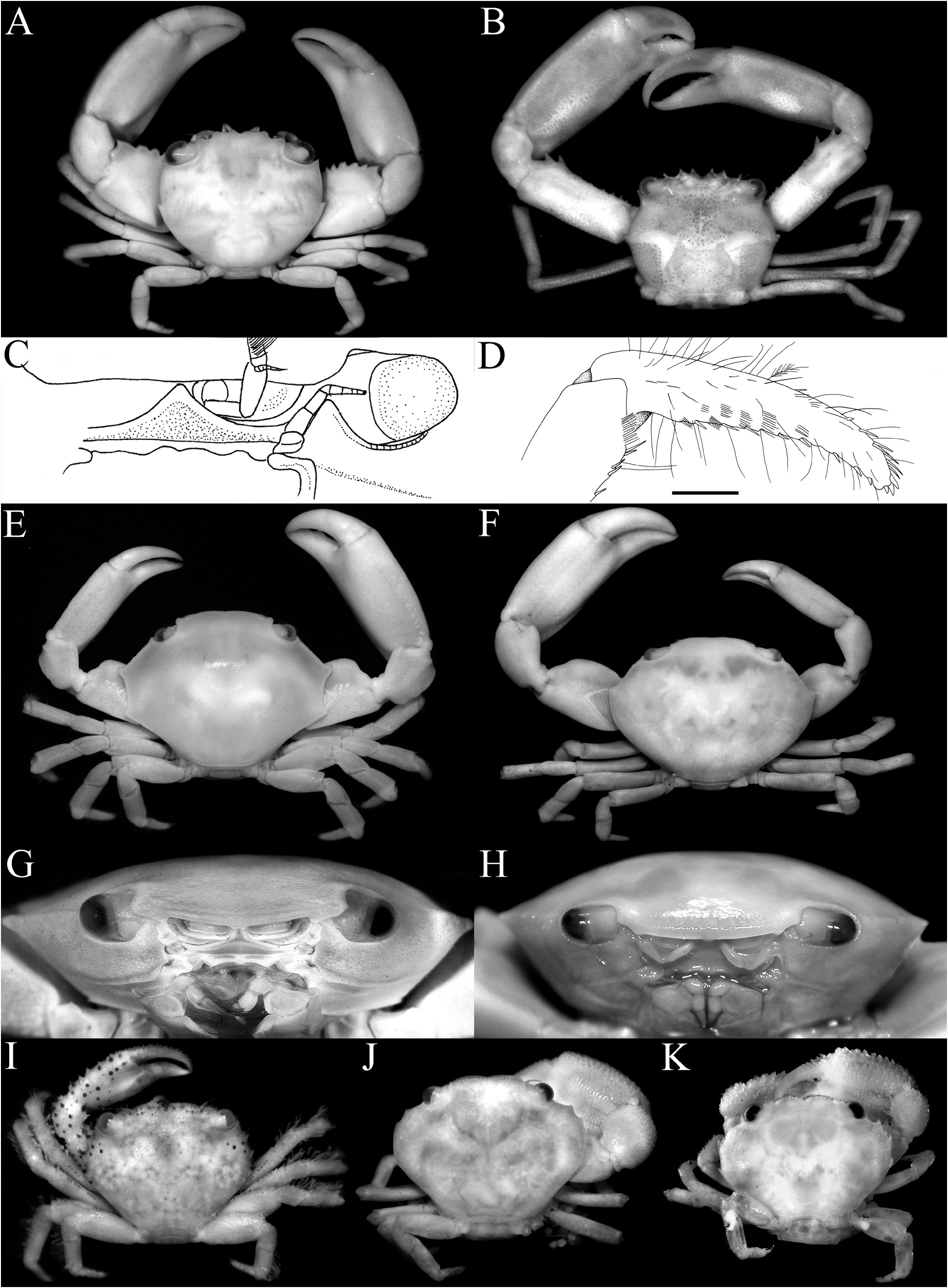
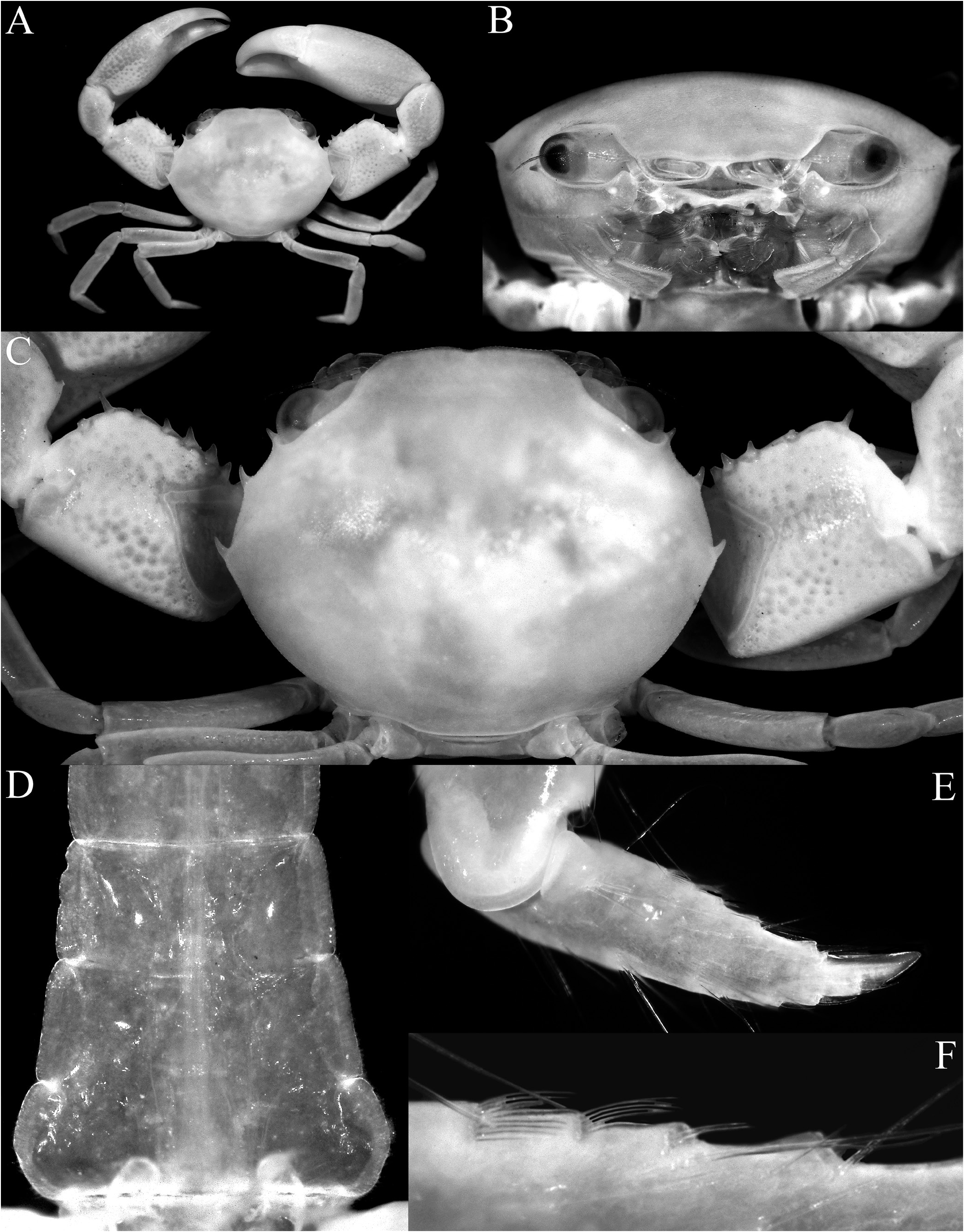
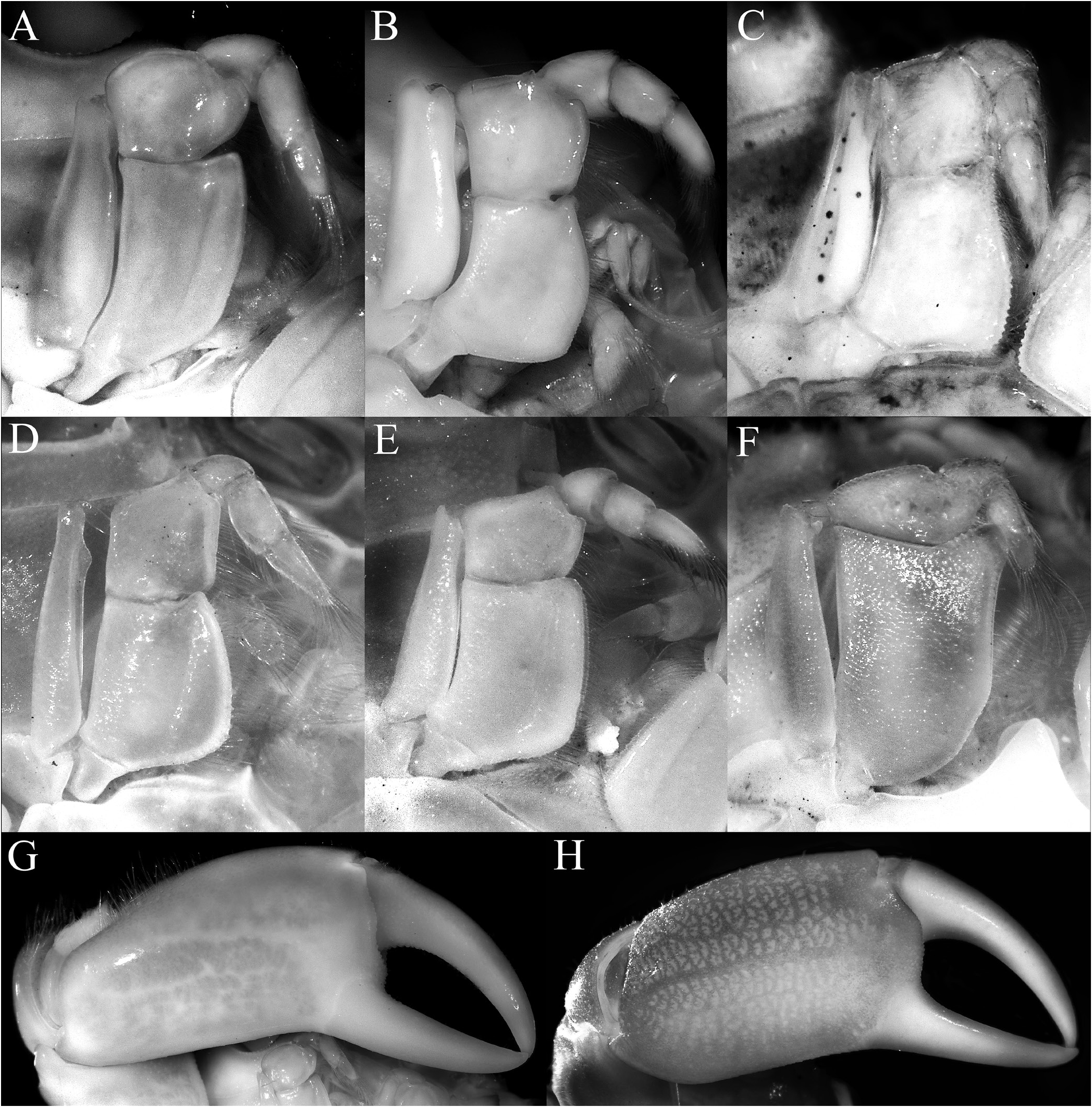
Diagnosis. Carapace transversely subovate, distinctly wider than long, widest near midlength ( Figs. 1A, B View Fig , 2A, B View Fig , 3A View Fig ); front gently sinuous to almost evenly curved; antero- and posterolateral margins clearly demarcated, anterolateral margin with 2 teeth ( Figs. 1A, B View Fig , 2A, B View Fig , 3A View Fig ); posterolateral margin convex ( Fig. 2A, B View Fig ); carapace relatively low, dorsal surface gently convex in frontal view ( Fig. 2C View Fig ); posterior margin of epistome with median lobe small, lateral margin relatively short, entire, gently concave, with small triangular lobe just before pterygostomial lobe, separated by fissure ( Figs. 2C View Fig , 3C View Fig ). Basal antennal article quadrate, mobile, peduncle entering hiatus of orbit. Maxilliped 3 merus subquadrate with anteroexternal part distinctly produced, auriculiform, wider than subrectangular ischium; distal edge of exopod reaching to distal edge of merus ( Fig. 3D View Fig ). Chelipeds heterochelous, relatively long, major cheliped about 1.7 times carapace width; merus quadrate in dorsal view, short, with sharp granule on subdistal edge of flexor margin, otherwise smooth, extensor margin smooth; carpus with strong tooth on inner angle ( Figs. 1A, B View Fig , 2D View Fig , 3A, E, F View Fig ). P2–5 short, merus relatively stout; dactylus margins with ungrouped, robust, movable spines, without comb-like rows of feeding setae ( Figs. 1A, B View Fig , 3A, H View Fig ). Sternopleonal cavity reaching anteriorly to level of midlength of P2 coxae but poorly demarcated ( Figs. 2E, F View Fig , 3G View Fig ); tubercle of male pleonal-locking mechanism small, on posterior edge of thoracic sternite 5, near suture with sternite 4 ( Figs. 2F View Fig , 3G View Fig ). Male pleon transversely narrow, lateral margins of somites 3–6 barely converging, all somites and telson free ( Figs. 2E View Fig , 3I View Fig ). G1 arcuate, relatively slender, gradually tapering distally, distal margins lined with spinules ( Fig. 4A–D View Fig ). G2 relatively slender, long, about four-fifths length of G1; distal portion prominent but short, filiform ( Fig. 4E View Fig ). Vulva flat, subcircular, on anterior part of sternite 6, adjacent to suture with sternite 5, positioned close to median line, covered by thin membrane ( Fig. 2H View Fig ).
Remarks. Rathbun (1898: 591) stated in her description of Ectaesthesius that the new genus was allied to Grapsillus MacLeay, 1838 , now a junior synonym of Trapezia Latreille, 1828 . Garth (1946: 467) commented that this “ Trapezia -like xanthid” must “occur with some frequency on the right type of bottom, which in this case was sand with some rock.”. Despite its external similarity to Trapezia , Ectaesthesius had been overlooked in considerations of Trapeziidae , probably because the genus is not known to be associated with cnidarians, sponges or other invertebrates and it is a monotypic genus that has only been rarely encountered. Poore & Ahyong (2023) tentatively placed the genus in Trapeziidae , but did not discuss the matter in detail. Whether Ectaesthesius is free-living or an obligate or facultative symbiont is not currently known (but see Remarks for Ectaesthesius ).
The complete fusion of male thoracic sternites 1–4 without any trace of sutures medially, the structure of the male pleon (with all the male pleonal somites free), and the elongated G2 excludes Ectaesthesius from Xanthidae (and Xanthoidea ) as is presently understood (see Lai et al., 2011; Mendoza et al., 2022). The suture between the male thoracic sternites 2 and 3 is demarcated in xanthids, and all have some male pleonal somites fused, even if the sutures may be visible.
In distinguishing the trapezioid families, the structure of the male pleon and the condition of the chelipeds have been used as key characters. According to Castro et al. (2004), all the male pleonal somites of tetraliids are free, but the male pleonal somites 3–5 are fused in trapeziids and domeciids. Whether the anterior margin of the merus of the cheliped is armed with sharp spines was a character used to distinguish trapezioid genera, although its phylogenetic value is debatable. Most trapezioids have the margin lined with sharp spines and spinules, but in the trapeziid Hexagonaloides Komai, Higashiji & Castro, 2010 , and in calocarcinids ( Calocarcinus Calman, 1909 , Philippicarcinus Garth & Kim, 1983 ), it is unarmed or almost so (cf. Takeda, 1980: fig. 1A; Garth & Kim, 1983: figs. 13a, 14a; Serène, 1984: pl. 42E, F; Galil & Clark, 1990: figs. 2f, g, 4e, g; Castro et al., 2004: pl. 4C, D; Komai et al., 2010: figs. 1, 3A, C). The anterior margin of the cheliped merus is smooth in Ectaesthesius except for a prominent granule on the distal angle of the ventral margin ( Figs. 1A, B View Fig , 2D View Fig , 3A View Fig ).
A key character present in many trapezioids is the presence of comb-like rows of mucus-gathering, or feeding, setae on the dactylus of P2–5 ( Galil, 1987). The setae vary from very prominent (with several distinct rows, e.g., Trapezia and Tetralia ) to less-developed conditions (e.g., in Quadrella , present only on the facial surfaces). It is absent in all Domeciidae ( Cherusius Low & Ng, 2012 , Domecia Eydoux & Souleyet, 1842 , Maldivia Borradaile, 1902 , and Palmyria Galil & Takeda, 1986 ) and Calocarcinidae ( Calocarcinus and Philippicarcinus ). Hexagonaloides was originally reported as lacking comb-like rows of feeding setae on P2–5 ( Komai et al., 2010) but Tomoyuki Komai kindly re-examined the type material of Hexagonaloides bathyalis Komai, Higashiji & Castro, 2010 and determined that this species actually possesses the comb-like rows of feeding setae ( Fig. 11D View Fig ), as in other quadrellines. The dactylus of P 2–5 in Calocarcinus and Philippicarcinus possesses robust, movable spines along the flexor margin, but there is no trace of any rows of comb-like feeding setae on the dactylus facial surface. This is a feature these two genera share with Ectaesthesius ( Fig. 3H View Fig ).
Castro et al. (2004) reported Sphenomerides as also lacking the comb-like rows of feeding setae (see also Serène, 1973, 1984), which is incorrect. Sphenomerides does also have comb-like rows of feeding setae on the P2–5 dactylus, albeit restricted to the flexor margin ( Castro, 2013) ( Fig. 12E, F View Fig ) (see Remarks for Sphenomeridinae ).
Tetraliids show marked heterochely in adults, with one chela much larger than the other (e.g., Castro et al., 2004: pl. 2A, B). The same is true for domeciids ( Castro et al., 2004: pl. 2A, B) and Ectaesthesius ( Figs. 1A, B View Fig , 3A, E, F View Fig ). In addition, the occlusal margins of the fingers of the chelipeds of adults are almost smooth and unarmed in most tetraliids (e.g., Figs. 1A, B View Fig , 6G, H View Fig ; Castro et al., 2004: pl. 2A, B), as in Ectaesthesius ( Figs. 1C, D View Fig , 2D View Fig , 3A, E, F View Fig ), although some species and smaller specimens of Tetralia Dana, 1851 , and Tetraloides Galil, 1986 have small teeth and denticles (e.g., Galil, 1986c: fig. 1B; Castro et al., 2004: pl. 1D, E). Domeciids also show marked heterochely, but the occlusal margins of their fingers are clearly blade-like and/or dentate ( Serène, 1984: pl. 43A, B; Galil & Takeda, 1986: figs. 3, 4, 7, 8). By contrast, other trapezioids usually have more homochelous chelipeds with the occlusal margins of the fingers dentate (e.g., Serène, 1984: pls. 38, 39, 41), although individuals of Sphenomerides , Calocarcinus , and Philippicarcinus are often heterochelous as well (e.g., Galil & Clark, 1990: fig. 6a; Castro et al., 2004: pl. 4C–E).
Serène (1984: 17) used the mouthparts to separate out Domeciidae , noting that the “third maxilliped has the merus very short and much broader than long. The second maxilliped has an endopod with the propodus and dactylus fused into a single, very large endite” (cf. Fig. 6F View Fig ; Galil & Takeda, 1986: figs. 2, 6). The propodus and dactylus of the endopod of the second maxilliped are free in trapeziids and tetraliids, and the maxilliped 3 merus is more quadrate. The maxillipeds 3 of trapeziids generally have an ischium that is subquadrate and the proximal margin is distinctly or slightly wider than the distal part (cf., Fig. 5B–D View Fig ; Galil, 1986a: figs. 1B, 3B; Galil & Clark, 1990: fig. 2c, Komai et al., 2010: fig. 2C; Lin & Ng, 2017: fig. 5B). In tetraliids, the maxilliped 3 ischium is distinctly longer and more rectangular, with the distal margin slightly wider than or subequal to the posterior margin ( Fig. 5A View Fig ; Galil, 1986b: fig. 2A, 1986c: fig. 2C; Galil & Clark, 1990: fig. 4c), resembling the condition in Ectaesthesius ( Fig. 3D View Fig ).
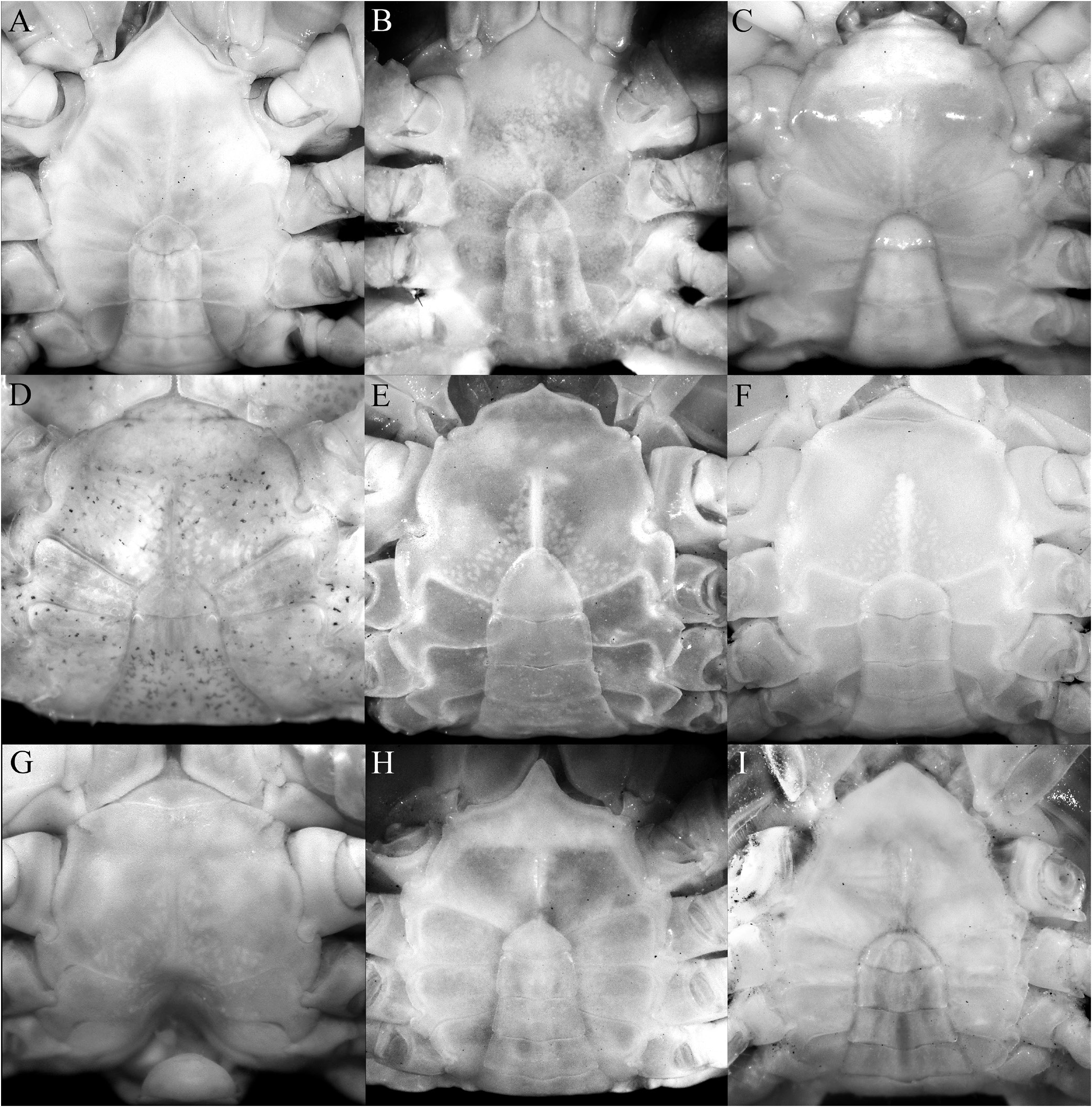
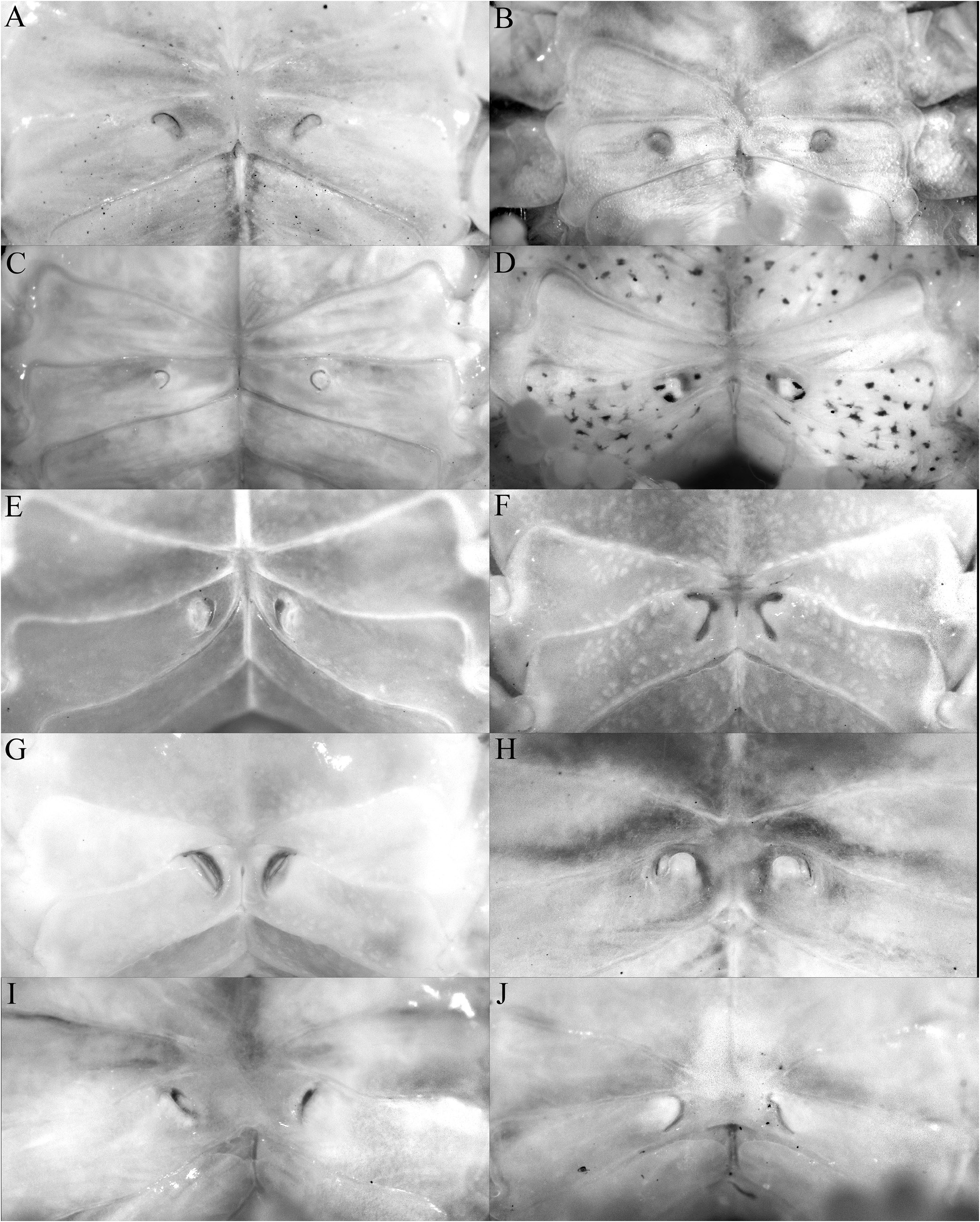
The shape of the posterior margin of the epistome is a character that does not appear to have been previously applied to the taxonomy of Trapezioidea , but we have found it helpful in distinguishing genera and subfamilies. The posterior margin of the epistome of Tetraliidae and Domeciidae has a low median lobe, with the lateral margin gently concave and entire or almost so, with only one lateral lobe just before the pterygostomial lobe, with the portions separated by a cleft ( Fig. 5A, E, F View Fig ). In Trapeziidae and Calocarcinidae , the posterior margin of the epistome has a distinct median lobe, with the lateral margin relatively short and gently concave, with a low triangular lobe just before the pterygostomial lobe ( Fig. 5B–D View Fig ; Komai et al., 2010: fig. 2B). The length of the lateral margin and strength of the lateral lobes differs between the three subfamilies recognised herein, but the structure is essentially the same. The structure of the posterior margin of the epistome of Ectaesthesius is similar to that of Tetraliidae and Domeciidae .
The condition of the male thoracic sternum, especially with regard to the degree of fusion between sternites 2 and 3 is important. While the male thoracic sternites 1 and 2 and the sternites 3 and 4 are completely fused in all trapezioids, the absence or presence of a suture between sternites 2 and 3 varies between families. Tetraliids and domeciids have no trace of a suture between male thoracic sternites 2 and 3, with sternites 1–4 forming one large plate ( Fig. 6A, B, H, I View Fig ; Galil, 1986b: fig. 2C, 1986c: fig. 2A; Galil & Takeda, 1986: figs. 1, 5). The suture between the male thoracic sternites 2 and 3 is deep and distinct in calocarcinids ( Fig. 6F, G View Fig ), and varies in trapeziids, from being deep and very distinct (as in Sphenomerides and most species of Trapezia ; Fig. 6C, E View Fig ), to having only the lateral sutures distinct with the median part shallower or less distinct (as in some species of Quadrella Dana, 1851b , Hexagonalia Galil, 1986a , and Trapezia ; Fig. 6D View Fig ; Galil, 1986a: fig. 1E, 3E; 1986b: fig. 2C) to absent, the separation between the sternites at most being demarcated by a gentle change in contour without any trace of a suture (as in Hexagonaloides and Sphenomerides ; Fig. 6E View Fig ; Komai et al., 2010: fig. 2D). The male sternopleonal cavity in trapezioids is generally shallow, but it is especially poorly demarcated in Ectaesthesius , with the margins being barely visible ( Figs. 2E, F View Fig , 3G View Fig ).
As discussed above, the condition of the male pleonal somites 3–5 (whether fused or articulated) is an important diagnostic feature separating the lineages within Trapezioidea . The male somites 3–5 are completely free in Tetraliidae ( Figs. 7A, B View Fig , 8A View Fig ; Galil, 1986b: fig. 2C, 1986c: fig. 2A; Castro et al., 2004: fig. 3A, B) and Domeciidae ( Figs. 7H, I View Fig , 8F View Fig ). Castro et al. (2004: 14, 17) incorrectly attributed fused male pleonal somites 3–5 to Domeciidae ; whereas we confirm here that they are not fused and all the somites are freely articulating in the four included genera (see also Serène, 1984: 291). Although Poore & Ahyong (2023) suggested that the male pleonal somites of Ectaesthesius might be fused but with sutures visible (as in some former trapeziids such as Philippicarcinus ), it is shown herein that all somites are freely articulating as reported by Garth (1946). The male somites 3–5 are fused to different degrees in Trapeziidae . Complete fusion, with at most only the lateral parts of the sutures still visible, is present in Hexagonalia , Hexagonaloides , Quadrella , and Trapezia as well as in Sphenomerides ( Figs. 7C–E View Fig , 8B–D View Fig ; Serène, 1984: fig. 190; Galil, 1986a: figs. 1E, 3E; Komai et al., 2010: fig. 2F). The presence of visible sutures between somites 3–5, sometimes relatively deep, but with the somites immovable except as a single plate, is evident in the former trapeziids, Calocarcinus and Philippicarcinus ( Figs. 7F, G View Fig , 8E View Fig ), herein placed in Calocarcinidae . The male pleon of Ectaesthesius is also especially narrow ( Fig. 3I View Fig ) compared with those of other trapezioids, which are distinctly wider and/or more triangular ( Fig. 7A–I View Fig ; Galil, 1986a: figs. 1E, 3E; Castro et al., 2004: fig. 3A; Komai et al., 2010: fig. 2F). The freely articulating male pleonal somites of Ectaesthesius bifrons , therefore, distinguishes it from Trapeziidae .
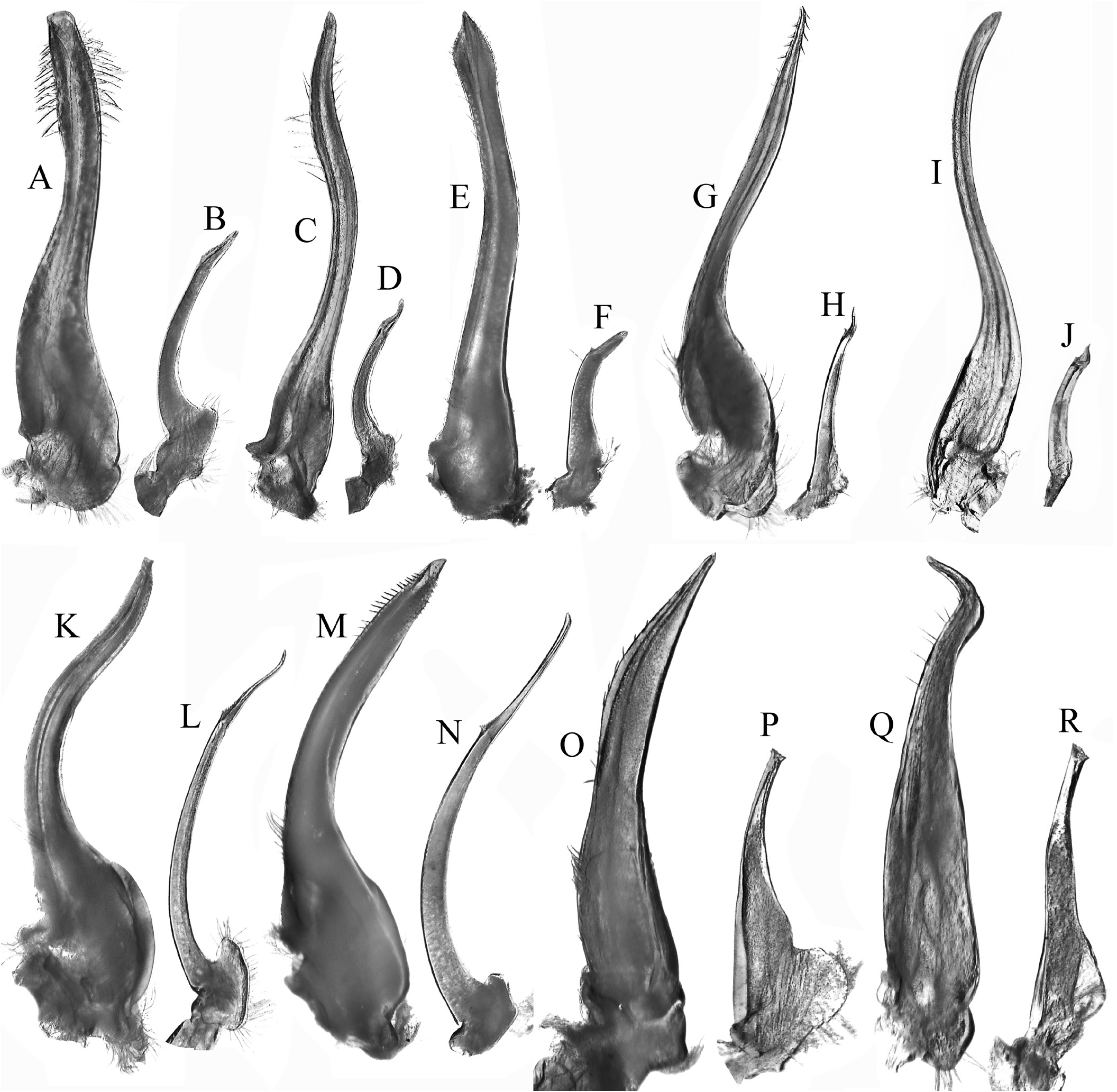
The structure and position of the male pleonal-locking mechanism, notably the tubercle on sternite 5, is of little value in separating the families. In the tetraliids Tetralia and Tetraloides , the tubercle is small and low, often indistinct, and always on the posterior edge of thoracic sternite 5 near the suture with sternite 6 ( Fig. 8G View Fig ). In the trapeziids Quadrella and Trapezia , the tubercle is on the posterior part of sternite 5, adjacent to and touching the suture with sternite 6 ( Fig. 8B, C View Fig ). In Sphenomerides , also a trapeziid, the tubercle is proportionally smaller but positioned on the anterior one-third of sternite 5 ( Fig. 8J View Fig ). In the calocarcinid, Calocarcinus , the tubercle is positioned on the posterior margin of sternite 5, just before the suture with sternite 6 ( Fig. 8K View Fig ), whereas in Philippicarcinus , also a calocarcinid, the tubercle is slightly more anterior but still on the posterior one-third of sternite 5. In the domeciid, Domecia , the tubercle is on the posterior margin of sternite 5, but not touching the suture with sternite 6 ( Fig. 8L View Fig ), whereas in another domeciid, Cherusius , the tubercle is on the posterior one-third of sternite 5. The tubercle on Ectaesthesius is most similar to that of tetraliids, with the structure being low and small, and positioned posteriorly on sternite 5 near the suture with sternite 6 ( Figs. 2F View Fig , 3G View Fig ). The G1 of trapezioids varies somewhat, sometimes even within one genus, and that of Ectaesthesius can be accommodated within this range of variation. The G1 of tetraliids is stout and straight in Tetralia ( Fig. 9A View Fig ; Serène, 1984: fig. 188; Galil & Clark, 1990: figs. 5c–f) and more slender and gently sinuous in Tetraloides ( Fig. 9C View Fig ; Serène, 1984: fig. 189; Galil, 1986b: fig. 2D). The G1 of Ectaesthesius is different from that of Tetralia and Tetraloides , being longer and arcuate with the tip slightly flared ( Fig. 4A–D View Fig ). The G1 varies substantially in form and size in domeciids ( Fig. 9O, Q View Fig ; Serène, 1984: figs. 201–203). The G1 of trapeziids varies substantially in form: stout like that of Tetralia in Trapezia ( Fig. 9E View Fig ; Serène, 1984: figs. 178–187; Castro, 1997a: fig. 2C, 1997b: fig. 4C); very slender and sinuous in Sphenomerides ( Fig. 7I View Fig ; Serène, 1973: figs. 27, 28, 1984: fig. 196); slender, long, and gently sinuous to straight, sometimes with long spines distally in Hexagonalia , Hexagonaloides , and Quadrella ( Fig. 7G View Fig ; Serène, 1984: figs. 191–195; Galil, 1997: fig. 3; Castro, 2005: fig. 2; Komai et al., 2010: fig. 2G; Lin & Ng, 2017: fig. 6A–D). The G 1 in calocarcinids is stout to relatively slender and sinuous in Calocarcinus ( Fig. 9K View Fig ; Takeda, 1980: 2 D; Serène, 1984: figs. 197, 198; Galil & Clark, 1990: figs. 1a–d, 3b–e), or stout and arcuate in Philippicarcinus ( Fig. 9M View Fig ; Garth & Kim, 1983: fig. 14c). The G2 of trapezioids varies widely. The G2 of tetraliids is relatively long, being half to one-third the length of the G1, but while the proximal portion is long, the distal portion is relatively short ( Fig. 9B, D View Fig ). The G2 of domeciids is diagnostic, being relatively stout and thick proximally, and with a cup-like apex instead of the slender, tapering, demarcated distal part ( Fig. 9P, R View Fig ). As in the G1, the G2 of trapeziids varies considerably in form; it is short (about one-quarter or one-fifth of the G1 length) in Hexagonaloides and Sphenomerides , with the constricted distal part very short and tapering ( Fig. 9J View Fig ; Komai et al., 2010: fig. 2H, I); with the proximal portion proportionally longer with a short distal part in Quadrella ( Fig. 9H View Fig ; Lin & Ng, 2017: fig. 6E, F); and in Trapezia , the G2 is about one-third as long as the G1 with a simple, straighter distal part. The G2 is proportionally much longer in calocarcinids ( Calocarcinus and Philippicarcinus ), with the proximal portion conspicuously long and the distal constricted portion elongated and filiform ( Fig. 9L, N View Fig ; Takeda, 1980: 2 E; Garth & Kim, 1983: fig. 14d; Serène, 1984: figs. 199, 200; Galil & Clark, 1990: figs. 1e, 3f). The G2 of Ectaesthesius is most similar to that of calocarcinids, being long and with the distal portion filiform and well demarcated ( Fig. 4E View Fig ).
Another character not previously used in trapezioid taxonomy is the structure of the vulvae. In tetraliids, the vulva is simple, flat, lunate to subcircular, positioned on the submedian part of sternite 6, separated relatively far apart, but with a flat lateral sternal vulvar cover and, as such, is an extension of the sternum, even if it is flexible and non-membranous ( Fig. 10A, B View Fig ). The vulvae are similar to those observed in Ectaesthesius except that, in addition to being covered by a sternal vulvar cover, the vulvae are positioned more anteriorly, adjacent to the suture with sternite 5, and closer to the median line ( Fig. 2H View Fig ). In Trapeziinae ( Trapezia ), the vulva closely resembles that of tetraliids in having a flat lateral, flexible, non-membranous sternal vulvar cover and positioned submedially on sternite 6 except that is proportionately smaller ( Fig. 10C View Fig ). In Quadrellinae and Sphenomeridinae , the vulva is more ovate, on the submedian part of sternite 6, and the two are positioned closer to the median line of the sternum, with the opening directed inwards and there is a prominent lateral sternal cover that arches over the opening but does not completely cover it, with the opening covered by a membrane ( Fig. 10D, E View Fig ). The vulva is proportionally larger in calocarcinids, varies from ovate to triangular, and is positioned on the anterior half of sternite 6, with the vulvae positioned closer to the median line and with the opening directed inwards. In Calocarcinus , the lateral sternal vulvar cover is large and rounded-triangular, leaving a relatively wide-open, crescentic space around it and the opening is covered by a membrane ( Fig. 10F View Fig ; Ahyong, 2009: fig. 1B). The vulvar cover is lower and subtruncate in shape in Philippicarcinus , leaving a wider, hemispherical opening that is covered by an obliquely positioned, thick membrane ( Fig. 10G View Fig ). Domeciid vulvae are all relatively large, on the anterior half of sternite 6, and positioned close to the median line, but vary in other details. It is directed anteriorly in Domecia , with the sternal vulvar cover large and directed anteriorly as well ( Fig. 10H View Fig ); relatively narrower and opened obliquely inwards and with a reduced, low vulvar cover in Cherusius ( Fig. 10I View Fig ); and similar to the preceding species but with a large and ovate vulvar cover in Palmyra ( Fig. 10J View Fig ).
In summary, with due consideration of the characters of Ectaesthesius discussed herein, we posit that Ectaesthesius is most closely allied to tetraliids, most conspicuously because all the male pleonal somites are free. In addition, the structure of the posterior margin of the epistome, although proportionately narrower than in tetraliids, is otherwise similar, with the lateral margins gently concave, entire, with only a triangular lobe just before the pterygostomial lobe ( Fig. 3C View Fig for Ectaesthesius ; Fig. 5A View Fig for tetraliids). The male anterior thoracic sternum is also of the same form in Ectaesthesius , Tetralia , and Tetraloides , with no trace of a suture between sternites 2 and 3 ( Fig. 2E View Fig for Ectaesthesius ; Fig. 7A, B View Fig for tetraliids). The major differences between Ectaesthesius and the two tetraliid genera are as follows. In Ectaesthesius , the carapace is transversely ovate, being wider than long ( Figs. 1A, B View Fig ; 2A, B View Fig ; 3A View Fig ) (longitudinally trapezoid-obpyriform in tetraliids; Fig. 1C, D View Fig ); the anterolateral margin of the carapace is long with two teeth ( Figs. 1A, B View Fig ; 2A, B View Fig ; 3A View Fig ) (vs short and unarmed in tetraliids; Fig. 1C, D View Fig ); the frontal margin of the carapace is smooth ( Fig. 2C View Fig ) (serrate in tetraliids; Fig. 5A View Fig ); the eyes are not inflated, with orbits correspondingly small ( Fig. 2A–C View Fig ) (large and globose in tetraliids; Fig. 1C, D View Fig ; 5A View Fig ); the basal antennal article is quadrate and short ( Fig. 2C View Fig ) (enlarged and wide in tetraliids; Fig. 5A View Fig ); the posterior margin of the epistome is proportionately narrower than in tetraliids ( Fig. 3C View Fig for Ectaesthesius ; Fig. 7A, B View Fig for tetraliids); the male anterior thoracic sternum is proportionately much wider ( Figs. 2A View Fig , 3G View Fig for Ectaesthesius ; Fig. 5A View Fig for tetraliids); the dactylus of P2–5 has robust movable spines ( Fig. 3H View Fig ), but no trace of any comb-like rows of feeding setae as in tetraliids (i.e., Galil, 1986b: fig. 3), perhaps an indication of a less specialised diet than in coral-mucus-feeding tetraliids; and the G2 length is proportionately about four-fifths that of G1, with an elongated filiform distal portion ( Fig. 4E View Fig ) (G2 half G1 length or less and with a short filiform distal portion in tetraliids; Fig. 9B, D View Fig ); and vulvae covered by thin membrane ( Fig. 2H View Fig ) (sternal vulvar cover present in tetraliids; Fig. 10A, B View Fig ). These differences support the recognition of a new family, Ectaesthesiidae , for Ectaesthesius .
The carapace of Ectaesthesius , in addition to that of tetraliids and some trapeziids, also superficially resembles that of the pilumnoid, Tanaocheles Kropp, 1984 . Kropp (1984) considered Tanaocheles to be a trapeziid, but Ng & Clark (2000) showed it to be a pilumnid and established Tanaochelinae for the two known species in the genus. The subfamily was later recognised as a distinct family in Pilumnoidea ( Ng et al., 2008). Tanaochelids, however, are markedly different from Ectaesthesius , with male thoracic sternites 2 and 3 separated by a deep suture ( Ng & Clark, 2000: fig. 1e), the G1 distinctly sinuous and slender without sharp spines on the lateral margins ( Ng & Clark, 2000: fig. 3a–c), the very short and sigmoid G2 ( Ng & Clark, 2000: fig. 3f), and the penis exiting at the tip of the condyle of the P5 coxa (see Ng & Clark, 2000: figs. 1e, 3a–f). In contrast, the male thoracic sternites 1–4 of Ectaesthesius are completely fused without sutures ( Fig. 2F View Fig , 3G View Fig ), the G1 is almost straight with rows of spines on the margins ( Fig. 4A–D View Fig ), the G2 is long with a well-developed distal part ( Fig. 4E View Fig ), and the penis exits at the base of the condyle of the P5 coxa.
It remains unclear if E. bifrons (and Ectaesthesiidae ) is free-living or associated with other invertebrates as accompanying specimen data did not record any associations. We must emphasise, however, that all the specimens known have been collected by dredge and they could easily have been dislodged from a host. As such, it is relevant that hydroids were collected together with E. bifrons at several localities ( Fraser, 1938, 1948; Calder et al., 2003) and ahermatypic scleractinian corals ( Endopachys grayi H. Milne Edwards & Haime, 1848 ) were collected together with E. bifrons from north of Española (= Hood) Island at Velero III station 814- 38 ( Durham & Barnard, 1952; Cairns, 1991), so a potential association is plausible. Even for the other trapezioids, the hosts of deep-water taxa, such as Philippicarcinus and Sphenomerides that are usually collected by dredge or trawl, were long unknown ( Castro et al., 2004), and were only recently confirmed to be sponges in both genera ( Komai et al., 2010; Castro, 2015), in addition to antipatharian corals in the case of the latter ( Silambarasan et al., 2023). Confirmation of the lifestyle of Ectaesthesius must await more definitive sampling observations.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.