Lysmata napoleoni, Grave & Anker, 2018
|
publication ID |
https://doi.org/10.11646/zootaxa.4429.2.13 |
|
publication LSID |
lsid:zoobank.org:pub:58F3C349-9521-4DAF-8A05-D3AC050779D6 |
|
DOI |
https://doi.org/10.5281/zenodo.5994493 |
|
persistent identifier |
https://treatment.plazi.org/id/038F191A-560E-FFFF-60BE-B61C886CFE3E |
|
treatment provided by |
Plazi |
|
scientific name |
Lysmata napoleoni |
| status |
sp. nov. |
Lysmata napoleoni sp. nov.
( Figs. 1–5 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 )
Lysmata sp.— Brown 2014: 57 (left photo).
Type material. St. Helena. Holotype: hermaphrodite (pocl 5.8 mm), off Long Ledge, -15.945° -5.753°, 15 m, leg. J. Brown & P. Wirtz, 14.I.2014, OUMNH .ZC.2018-04-001 (fcn 140114 /60/1&2). Paratypes: ov. hermaphrodite (pocl 4.0 mm), James Bay Wharf steps, -15.921° -5.718°, 9.8 m, leg. J. Brown, 06.IX.2013, OUMNH.ZC. 2018-04- 0 0 2 (fcn 130906 /44/11); ov. hermaphrodite (pocl 3.8 mm), same location, 9.7 m, leg. J. Brown, 28.III.2013, OUMNH . ZC.2018-04-003 (fcn 130328 /Q4/05); 4 hermaphrodites (pocl 3.5–5.0 mm), Buoys Hole , -15.911° - 5.688°, 17.7 m, leg. J. Brown & P. Wirtz, 19.I.2014, OUMNH . ZC.2018-04-004 (fcn 140119 /67/ 01-02-05); 1 hermaphrodite (pocl 4.1 mm), Papa Nui wreck, -15.922° -5.72°, leg. P. Wirtz & J. Brown, 23.I.2014, OUMNH .ZC.2018-04-005 (fcn 140123 /74/03); 1 ov. hermaphrodite (pocl 5.2 mm, dissected), same collection data, MZUSP 37298 (fcn 140123 /74/04).
Non-type material. Ascension Island. 1 ov. hermaphrodite (pocl 5.2 mm), 1 hermaphrodite (pocl 3.6 mm), Two Hook dive site, 15 m, leg. P. Wirtz, 16.VII.2015, OUMNH .ZC.2018-01-024; 1 hermaphrodite (pocl 3.6 m), Red Rock, -7.89435° -14.39475°, 8.5 m, leg. A. Richardson, 20.VII.2015, OUMNH.ZC.2018-01-025 (fcn C20 009).
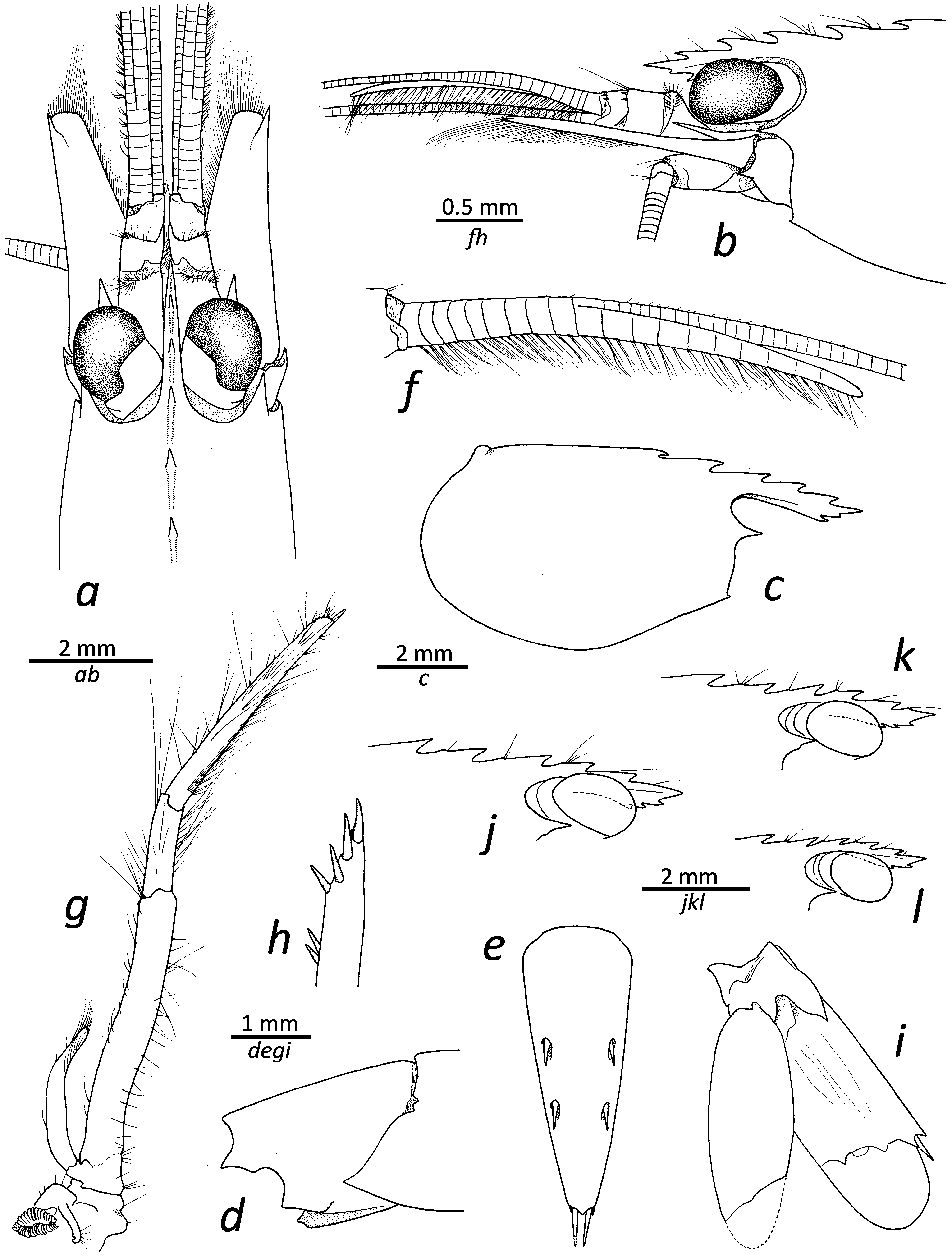
Description. Rostrum ( Fig. 1a, b, j–l View FIGURE 1 ) elongate, stout, about four times as long as carapace, reaching midlength of second article of antennular peduncle; dorsal margin with five teeth ( holotype), most posterior tooth at about 0.25 of carapace length, third tooth close to post-orbital margin or slightly more anterior; ventral margin with two or three teeth (three in holotype), relatively distal, tip not subdivided. Carapace smooth, with rounded posteroventral margin ( Fig. 1c View FIGURE 1 ); pterygostomial angle with small, acute tooth ( Fig. 1b View FIGURE 1 ); antennal tooth strong, acute, falling short of middle of cornea ( Fig. 1a–c View FIGURE 1 ).
First three pleonites ventrally rounded; fourth somite with square (about 90°) posterolateral angle; fifth somite ( Fig. 1d View FIGURE 1 ) with well-developed, distally projecting posterolateral angle; sixth somite about 1.5 as long as fifth, with square posteroventral angle ( Fig. 1d View FIGURE 1 ); posterolateral margin of sixth pleuron with well-developed polygonal lobe above articulation with uropod, posteromedial part developed into broad tooth. Telson ( Fig. 1e View FIGURE 1 ) about 2.5 times as long as wide, tapering posteriorly; dorsal surface with two pairs of spines, approximately inserted at 0.4 and 0.6 of telson length, respectively ( holotype); posterior margin medially bluntly produced, furnished with two pairs of spiniform setae, mesial pair about four times as long as lateral pair.
Antennular peduncle ( Fig. 1a, b View FIGURE 1 ) about 0.7 times as long as scaphocerite; first article about 2.2–2.5 times as long as second; third article about half as long as second; distodorsal margins of second and third articles furnished with few small spinules; stylocerite reaching distal margin of first article; lateral antennular flagellum long; accessory branch ( Fig. 1f View FIGURE 1 ) with 11 free divisions ( holotype), fused portion with nine divisions ( holotype), free portion about 1.7 times as long as fused portion ( holotype). Scaphocerite about 3.5 times as long as wide; lateral margin straight; distolateral tooth overreaching distal margin of blade.
Third maxilliped ( Fig. 1g View FIGURE 1 ) overreaching scaphocerite by distal half of ultimate article; exopod about 0.5 times as long as antepenultimate article of endopod; penultimate article about 0.4 times as long as ultimate article; tip with three subdistal and one distal spiniform setae.
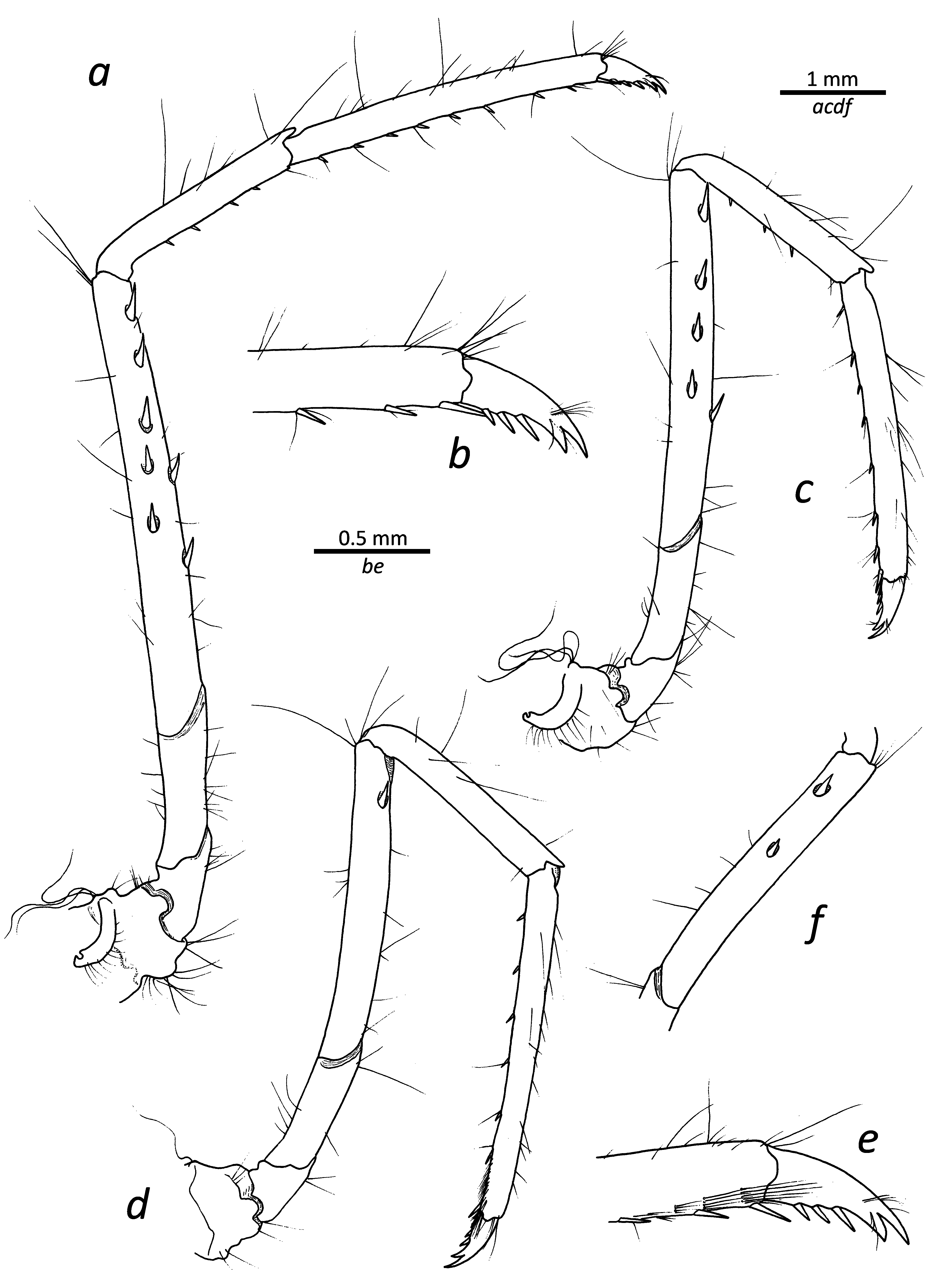
First pereiopod ( Fig. 2a–c View FIGURE 2 ) reaching past scaphocerite by length of fingers when extended; ischium with row of spinules along ventral margin; merus about 1.2 times as long as carpus; chela about 1.2 times as long as carpus, with palm about 1.6 times as long as fingers; tips of both pollex and dactylus simple; carpo-propodal brush well developed.
Second pereiopod ( Fig. 2d, e View FIGURE 2 ) slender, when fully extended overreaching scaphocerite by distal 2/3 of carpus, ending in small, simple chelae; right and left pereiopod subequal, left second pereiopod proportionally longer than right by only about 0.2 of length ( holotype); ischium with two–three weak, distal annulations; merus subdivided into 14 (right) and 15 (left) divisions ( holotype); carpus twice as long as merus, subdivided into 24 (right) and 25 (left) divisions ( holotype).
Third to fifth pereiopods generally similar, decreasing in length ( Fig. 3 View FIGURE 3 ). Third pereiopod ( Fig. 3a, b View FIGURE 3 ) overreaching scaphocerite by entire length of propodus; merus about 3.4 times as long as ischium, mesially with five spines ( holotype) and additional two spines present along ventral margin on right side, six and three, respectively, spines on left side; carpus about half as long as merus, with four spiniform setae on ventral margin ( holotype); propodus about 1.4 times as long as carpus, ventrally armed ( holotype) with six spiniform setae on right side, nine on left side, distal of those paired; dactylus about 0.2 as long as propodus, biunguiculate, with dorsal unguis larger than ventral one; flexor margin with additional three spinules. Fourth pereiopod ( Fig. 3c View FIGURE 3 ) generally similar to third, with four spines in mesial series on merus ( holotype, both sides) and single ventral one. Fifth pereiopod ( Fig. 3d–f View FIGURE 3 ) similar, with one (right) or two (left) spines in mesial series on merus ( holotype).
Uropod ( Fig. 1i View FIGURE 1 ) with relatively slender exopod and endopod, latter with well-developed diaeresis bearing three spaced acute or subacute teeth, lateral-most strongest, distolateral tooth and adjacent spiniform seta both strong.
Colour pattern ( Figs. 4 View FIGURE 4 , 5 View FIGURE 5 ): body mostly deep red, except some semi-transparent areas on anterior carapace flanks, two conspicuous white transverse bands, both quite irregular in shape, one running across posterior margin of carapace and another across middle of third pleonite, as well as series of white spots or patches of various sizes, on dorsal surface of carapace and on pleon, in latter case typically arranged in transverse row of spaced spots (for example, as dorsal, subdorsal, lateral and ventrolateral), or forming two continuing bands running ventrolaterally on each side of pleon, from third pleomere to sixth pleonite; ventral base of each eye peduncle and area between them also white; antennular and antennal peduncles, their flagella, first to fifth pereiopods and tail fan mostly deep red or reddish, with occasional larger white spots or bands, latter especially well marked on telson and uropodal margin; fresh eggs orange-red.
Morphological variation. In terms of rostral variation, L. napoleoni sp. nov. exhibits little variation, with all examined specimens possessing five dorsal teeth, although the position of the second and third proximal teeth differs slightly between specimens; approximately half of the specimens possess two ventral teeth, the other half have three ( Fig. 1c, j–l View FIGURE 1 ). The length of the free versus fused portion of the accessory branch of the antennal flagellum varies between 1.1 and 2.2, with a median value of about 1.7; the number of divisions in the free part varies between 10 and 13, whereas the number of divisions in the fused part varies between 6 and 9.
The comparative lengths of the left and right second pereiopods differs slightly, with sometimes one being longer (left or right), and on occasion both being of approximately the same length. In terms of subdivision of the second pereiopod, the number of divisions of the merus ranges from 13 to 18, with less variation seen in the carpus (25–27). Typically, there is a slight difference (1–2 divisions) between the left and the right sides. The number of spines on the merus (combined for mesial and ventral series) varies significantly on the third pereiopod (3–9) with one individual having no spines at all. Less variation in this feature exists on the fourth (2–5) and fifth (1–2) pereiopods. The number of ventral spiniform setae on the third pereiopod propodus is also not constant, ranging from 6 to 10, with the distal one always being paired
Etymology. The species is named after Napoléon Bonaparte ( 1769–1821), arguably St. Helena’s most famous resident, from his exile to the island in 1815 to his death there in 1821.
Type locality. St. Helena Island, south-central Atlantic.
Distribution. Currently, the species is known only from St. Helena and Ascension Island, in the south-central Atlantic Ocean.
Ecology. All specimens of Lysmata napoleoni sp. nov. were collected while scuba diving at 8.5–17.7 m, on hard or mixed (rocky-sandy) bottoms. The new species appears to be always associated with – or at least is found in the vicinity of – the sea anemone Telmatactis cricoides (Duchassaing) (P. Wirtz, pers. comm.), although the exact nature of this association remains unknown. Photographic evidence ( Fig. 5b View FIGURE 5 ) shows that the new species engages in nocturnal fish cleaning behaviour (see also Brown et al. 2017).
Taxonomic remarks. On account of the length of the free portion of the accessory branch of the lateral antennular flagellum, Lysmata napoleoni sp. nov. belongs to the so-called “long-branch group” of the genus Lysmata ( Fiedler et al. 2010) , which at least partly corresponds to the “cosmopolitan clade” in the phylogeny of Baeza (2010). In the eastern Atlantic, only three further species belong to this clade, viz. L. moorei , L. nilita Dohrn & Holthuis, 1950 and L. seticaudata . The western Atlantic L. intermedia ( Kingsley, 1879) and L. jundalini Rhyne, Calado & dos Santos, 2012 also belong to this clade ( d’Udekem d’Acoz 2000; Rhyne et al. 2012). All remaining Atlantic species of Lysmata can be easily differentiated from the new species by their very short or unguiform accessory branch (see Anker & Cox 2011; Prakash & Baeza 2017).
Morphologically, Lysmata napoleoni sp. nov. may be separated from both L. intermedia and L. jundalini by the number of fused and free divisions in the accessory branch of the lateral antennular flagellum, which is 17–18 (fused) and 3–4 (free) in L. intermedia , and 18–24 (fused) and 3 (free) in L. jundalini , versus 10–13 (free) and 6–9 (fused) in L. napoleoni sp. nov., as well as by the relative proportions of these two parts, with the free part being less than 0.15 of the fused part in both L. intermedia and L. jundalini vs. being much longer in L. napoleoni sp. nov. (1.15–2.17) (see d’Udekem d’Acoz 2000; Rhyne et al. 2012). On the same basis, L. napoleoni sp. nov. can be distinguished from L. nilita , in which the free part of the accessory branch is less than 0.33 of the fused part ( Dohrn & Holthuis 1950).
Lysmata moorei is a relatively poorly described species, which shows considerable variation in size and morphology between the western, central and eastern Atlantic populations (De Grave & Anker, unpublished data). Although L. moorei exhibits similar proportions of the fused vs. free part of the accessory branch to the new species, a number of other morphological characteristics clearly differentiate the two species. In L. moorei , the scaphocerite is relatively short and wide, with a short distolateral tooth, not overreaching the distal margin of the blade ( Rathbun 1901), whereas in L. napoleoni sp. nov., it is rather elongate and narrow, with the distolateral tooth clearly overreaching the blade ( Fig. 1a View FIGURE 1 ). The number of carpal divisions in the second pereiopod is about 17 ( Rathbun 1901) vs. considerably higher (25–27) in the new species ( Fig. 2d View FIGURE 2 ). In addition, only a single post-orbital tooth is present in L. moorei ( Rathbun 1901; Fransen 1991), whilst two are present in L. napoleoni sp. nov. ( Fig. 1b View FIGURE 1 ). In the material of L. moorei from Cape Verde, these features are confirmed: all specimens harbour only a single post-orbital tooth, a relatively short scaphocerite, with the distal tooth not overreaching the blade, and the second pereiopod with 16-17 carpal divisions.
In overall morphology, Lysmata napoleoni sp. nov. appears to be closest to the widely distributed L. seticaudata , for which there are surprisingly few good illustrations in recent taxonomic literature (e.g. Dohrn & Holthuis 1950; Zariquiey-Álvarez 1968; d’Udekem d’Acoz 2000). Nevertheless, based on rostral morphology, proportions of the scaphocerite, as well as the armature of the ambulatory pereiopods, L. napoleoni sp. nov. and L. seticaudata appear to be very similar. Although the drawings of the antennule of L. seticaudata in Dohrn & Holthuis (1950) and d’Udekem d’Acoz (2000) are somewhat conflicting, they agree in that the number of divisions in the fixed part of the accessory branch of the antennule is 15 or more, contrasting to 6–9 in the new species. This distinguishing feature is herein confirmed using a large sample from Corsica, in which the number of carpal divisions ranges from 19 to 27, thus allowing a morphological separation of the two taxa.
Lysmata napoleoni sp. nov. displays a striking colour pattern ( Figs. 4 View FIGURE 4 , 5 View FIGURE 5 ) and may be distinguished at once from all Atlantic congeners for which the colour pattern is known. For instance, the colour pattern of the morphologically closest Atlantic species, L. seticaudata (see photograph in González Pérez 1995) and L. moorei (see photograph in Brown 2015) are quite distinct from that of the new species. It is important to note that the colour patterns of several Atlantic species of Lysmata ( L. uncicornis Holthuis & Maurin, 1952 ; L. anchisteus Chace, 1972 ; L. stenolepis Crosnier & Forest, 1973 ; L. baueri Prakash & Baeza 2017 ) are presently unknown. These, however do not belong to the “long-branch group” and therefore, can be separated from L. napoleoni sp. nov. on the basis of the antennular morphology alone. Nevertheless, an effort should be made in the future to recollect and document the colour pattern of these species to facilitate easy field recognition.
Discussion. The present description raises to four the number of Lysmata species known from the isolated islands in the south-central Atlantic. This includes the record of L. intermedia from two locations at Ascension Island by Manning & Chace (1990), which was later corrected to “probably L. seticaudata ” in d’Udekem d’Acoz (2000) . Despite extensive fieldwork by several teams on Ascension Island, forming the basis of the revised checklist published by De Grave et al. (2017), no specimens identifiable as L. seticaudata were encountered. In addition, no records exist for L. seticaudata south of the Canary Islands ( d’Udekem d’Acoz 2000). Given the close morphological similarity between L. seticaudata and L. napoleoni sp. nov., and in the absence of any colour notes for the two specimens forming the basis of the record from Ascension Island, we postulate that the material reported by Manning & Chace (1990) as L. intermedia may potentially refer to the present new species.
Colour patterns are of high taxonomic value in the genus Lysmata , and are largely species specific, representing the most practical and easiest field diagnostic character in most groups (Fielder et al. 2010). Therefore, we advise against further descriptions of new taxa in Lysmata without colour pattern information, unless they exhibit truly unique species-level morphological differences. We also council against erecting new species based on single specimens (e.g. Gan & Li 2016; Prakash & Baeza 2017; Wang & Sha 2018), as has equally been done in recent years. All Lysmata species are rather variable in the traditional morphological characters used to differentiate them, such as the meral spination on the ambulatory pereiopods and the number of carpal divisions the second pereiopod, as amply demonstrated by the type series of L. napoleoni . A large part of future taxonomic work in Lysmata will be trying to match these “ mihi itch ” descriptions (see Evenhuis 2008) with newly collected, sequenceable and photo-vouchered material, to confirm the validity of these taxa.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Lysmata napoleoni
| Grave, Sammy De & Anker, Arthur 2018 |
Lysmata
| Brown 2014 : 57 |