Resomia convoluta ( Moser, 1925 )
|
publication ID |
https://doi.org/10.5281/zenodo.174440 |
|
DOI |
https://doi.org/10.5281/zenodo.5688261 |
|
persistent identifier |
https://treatment.plazi.org/id/038F8C6C-FFDF-D42F-6314-FB4C4ED8AFAC |
|
treatment provided by |
Plazi |
|
scientific name |
Resomia convoluta ( Moser, 1925 ) |
| status |
|
Resomia convoluta ( Moser, 1925) View in CoL
Diagnosis: Very young nectophores bearing no lateral digitate processes. Bracts with relatively small, triangular distal facet on upper surface; with bracteal canal ending at distal point below a cluster of nematocysts. Palpons without distal cluster of nematocysts.
Material examined: Two specimens kindly loaned to me by Dr Francesc Pagès collected in the Weddell Sea (see Pagès and Kurbjeweit 1994) by the RV Polarstern during the Antarktis IX/2 cruise at Stations 67 ( 2.xii.1990, 66°27.9’S, 28°43.9’W) and 94 ( 10.xii. 1990, 68°49.1’S, 17°55.5’W). Both specimens were collected in the 1000– 500m depth range using a multiple opening/closing net system ( 0.25 m 2 mouth opening, 100µm mesh).
Description: The description is based mainly on the specimen captured at St. 67, as the one from St. 94 is in relatively poor condition.
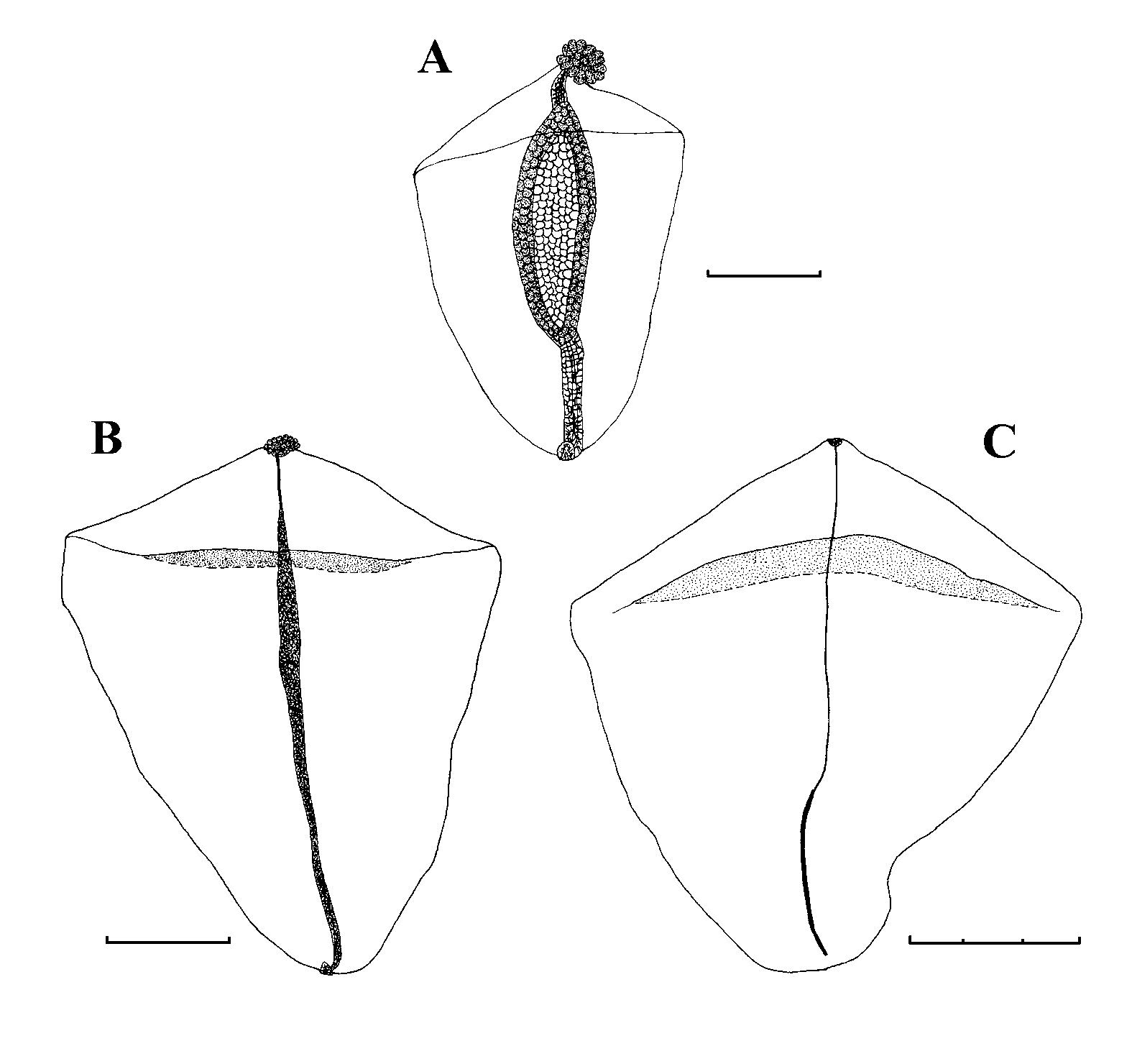
Pneumatophore: The pneumatophore of the specimen from St. 67 ( Fig. 4 View FIGURE 4 A) measured 1.88 mm in height and 0.55 mm in maximum diameter. It was quite featureless, apart from a darker patch of cells at the apex, which may have been pigmented in life. There had been some distortion as the base of the gas gland did not reach the base of the pneumatophore. The pneumatosaccus of the pneumatophore of the specimen from St. 94 ( Fig. 4 View FIGURE 4 B) had clearly exploded due to gas expansion whilst being brought to the surface, as the expanded gas can be seen within the nectosome. It was greatly distorted and measured 2 mm in length and 0.95 mm in maximum diameter. The significance of this distortion is discussed below.
Nectosome: Buds of nectophores and a single young one were attached at the base of the pneumatophore on the specimen from St. 67 ( Fig. 4 View FIGURE 4 A), on the ventral side of the stem.
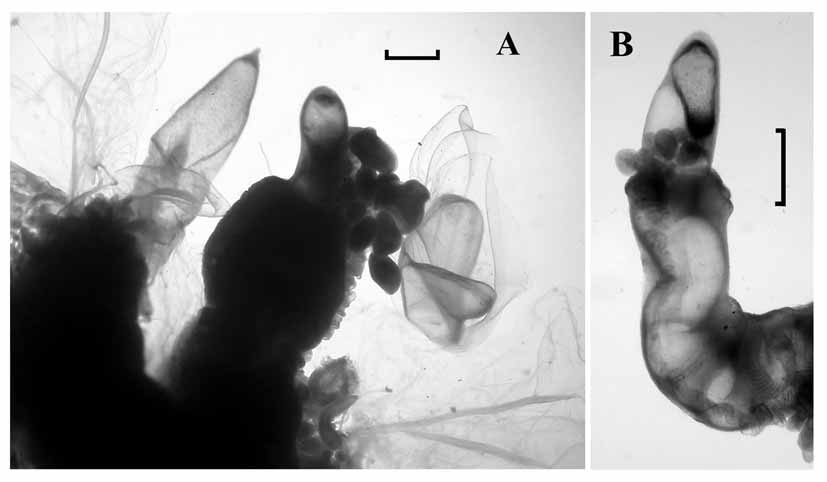
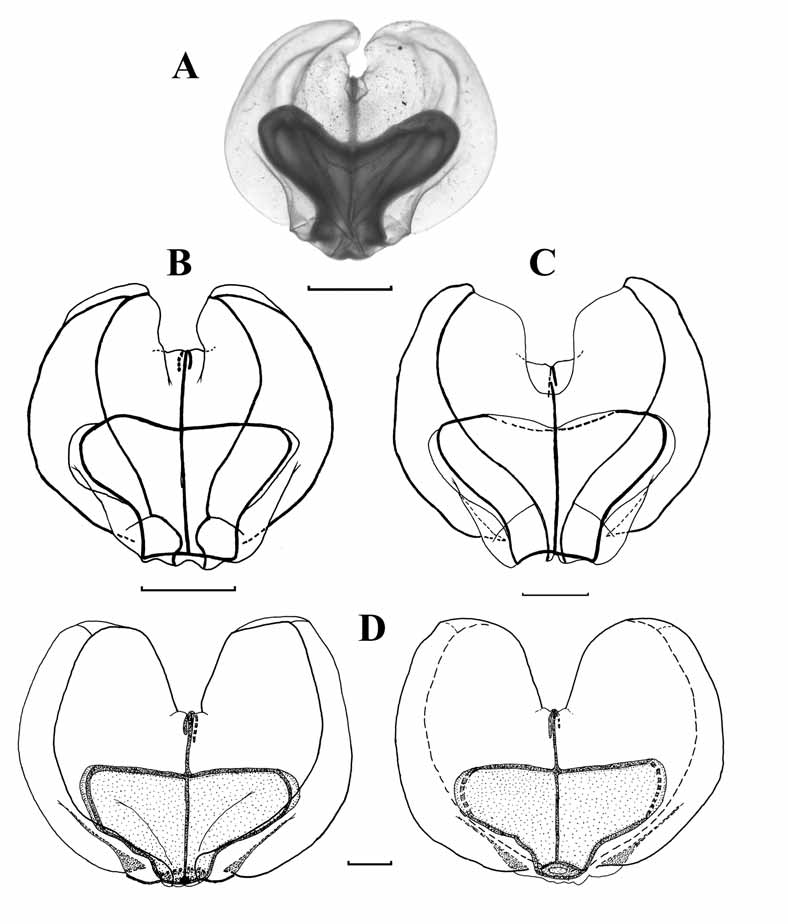
Nectophore: Twenty six nectophores, at various stages of development, were found with the St. 67 specimen. They measured up to 15.7 mm in width and 13 mm in length. A photograph of a young one ( 3.1 mm wide, 2.8 mm long) is shown in Fig. 5 View FIGURE 5 A. None of these young nectophores showed any signs of lateral digitate processes, in contrast to those of Resomia similis (see Fig. 13 View FIGURE 13 ). Slightly larger nectophores were found with the St. 94 specimen ( Fig. 5 View FIGURE 5 B, C). These clearly show the upper and lower lateral ridges. The upper facet is much smaller than the lower one, and the pairs of ridges define a broad, oblique lateral facet. As the upper laterals curved in toward the mid-line they each divided off a short, weak lateral ridge that petered out on the lateral process. The upper laterals then continued to the ostium, leaving a deep median furrow between them.
As the nectophores develop further they gradually take on the typical heart-shaped appearance of the young nectophores described by Moser (1925). The young nectophore shown in Fig. 5 View FIGURE 5 D, which measured 5.6 mm in length and 7 mm in width, clearly showed pairs of upper and lower lateral ridges that united close to the tips of the axial wings, with a narrow, concave, oblique lateral facet between them; the upper facet still being smaller than the lower one. The upper lateral ridges remained far apart for most of their lengths before curving in to join the ostium, leaving a narrow, but quite deep, median furrow between them. The lower lateral ridges petered out on the lower side of the nectophore marginally before reaching the ostium. Slightly swollen lateral processes extended out from the ostium, and then axially along the middle of the lateral facets for a short distance. These processes bore narrow strips of ectodermal cells on their surfaces that, in common with many other species, were probably sites of bioluminescence. As the nectophores enlarged and the upper lateral ridges moved further and further apart, the lateral branches that they gave off axial to the ostium became less and less apparent, such that at the stage shown in Fig. 5 View FIGURE 5 D no trace of them could be discerned.
In these maturing nectophores ( Fig. 5 View FIGURE 5 D) the nectosac occupied only the ostial third to half of the nectophore. Its axial surface was flat, and there was no sign of any muscle-free area. The ostial opening was quite small, and slightly displaced onto the lower side of the nectophore. There was a minute thrust block over which the pallial canal ran, from the upper to the lower surface of the nectophore. Both ascending and descending pallial canals were present, and of approximately equal length. The pedicular canal arose slightly on the lower side of the nectophore and ran straight to the nectosac. There it gave rise directly to the four radial canals, all of which had straight courses to the ostial ring canal.
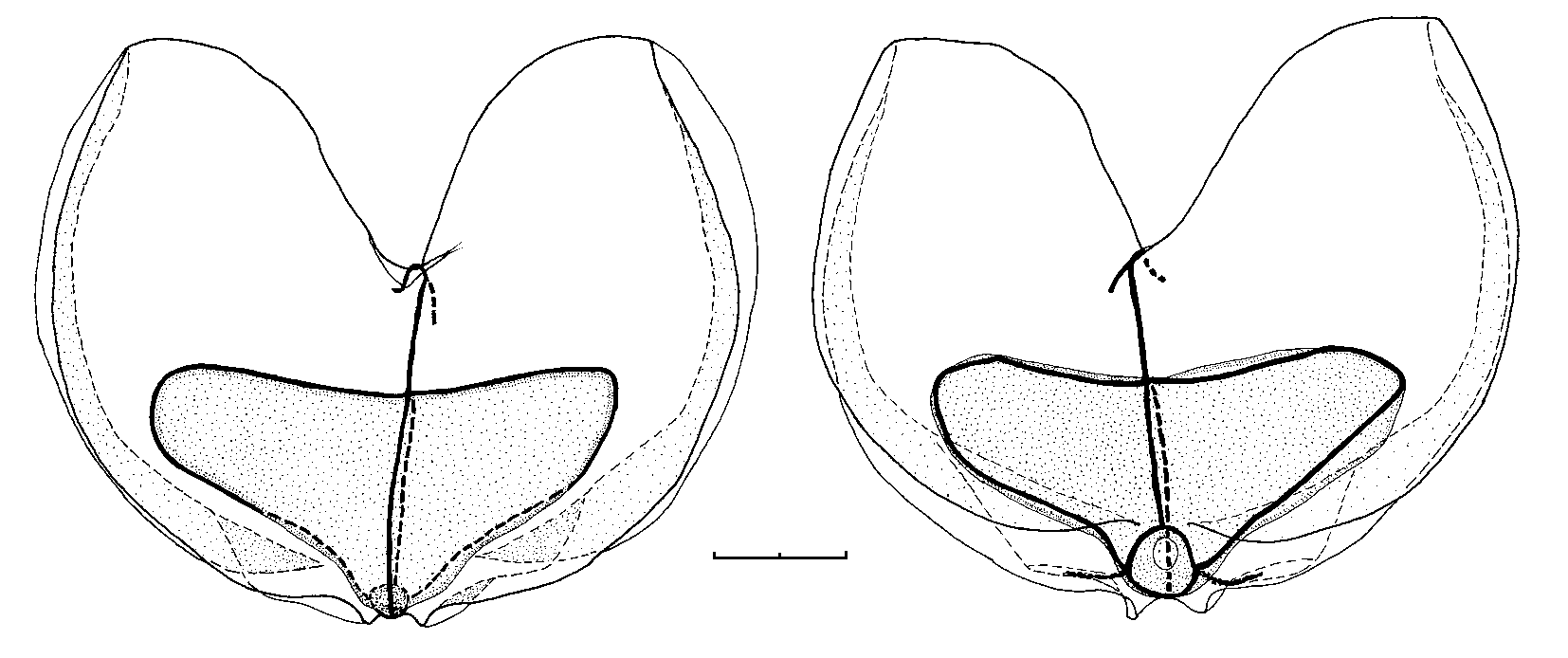
An almost fully developed nectophore, 8 mm long and 10.8 mm wide, is shown in Fig. 6 View FIGURE 6 . The upper and lower sides were of approximately equal width, with deeply concave lateral facets between the pairs of upper and lower lateral ridges. However, it is likely that this concavity is a preservation artefact. The lower lateral ridges continued to peter out on the lower surface of the nectophore just before reaching the ostium, although in some cases they appeared to reach it. There was no trace whatsoever of the lateral branches from the upper lateral ridges as noted in the youngest nectophores. The lateral processes from the ostium were slightly less prominent than in the younger nectophores, but still very noticeable were the narrow strips of ectodermal cells passing outwards from the points where the lateral radial canals met the ostial ring canal. The nectosac still extended to less than half the length of the nectophore, and its axial surface was flat and without any trace of a muscle-free zone.
Up to this stage the nectophores had a fairly rigid appearance, and the ridges were clearly distinguishable. However, as they enlarged further, the mesogloea, particularly in the axial wings, became more flaccid, and the ridge pattern less pronounced, although it still could be easily discerned in the well-preserved material available. These large flimsy nectophores were easily damaged. On some there was a semblance of a partial vertical lateral ridge between the upper and lower laterals. However, this was considered to be a fold caused by shrinkage or distortion during preservation.
Siphosome:
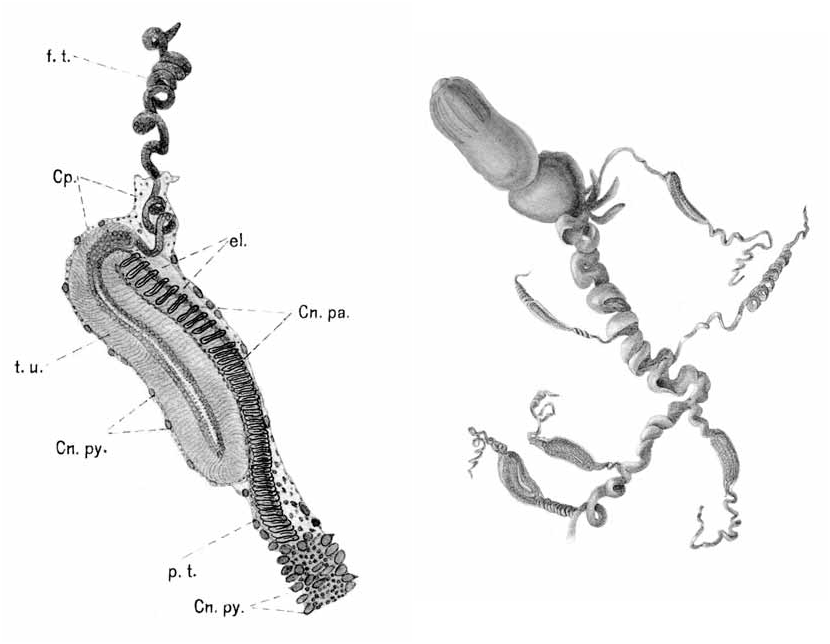
Bract: ( Fig. 7 View FIGURE 7 ). The bracts were quadrangular in shape, with rounded corners, and measured up to 10 mm in length and 8 mm in greatest width. The largest bracts were very flimsy. There was a transverse ridge across the upper side of the bract close to its distal end that delineated a triangular distal facet. In the younger bracts ( Fig. 7 View FIGURE 7 A), this ridge stretched from one side of the bract to the other, but did not overhang the distal facet. On intermediate sized bracts ( Fig. 7 View FIGURE 7 B) the connections to the sides of the bract were weak or totally absent, and the central part of the ridge slightly overhung the distal facet. In the largest bracts ( Fig. 7 View FIGURE 7 (C)) the transverse ridge usually did not connect with the sides of the bract, while it overhung the distal facet more extensively. The bract was thickest in the middle region of the transverse ridge and tapered down gradually toward the proximal end. Toward the distal end the bract rapidly lost depth on its upper side, but not on its lower one.
In the youngest bracts, the proximal end of the bracteal canal extended a short distance on to the upper side of the bract, before running over on to the lower side and then directly to the distal tip. It terminated below a distinctive cluster of nematocysts. The canal had very thick walls. At its proximal and distal ends the canal was narrow, but in its middle region its cavity was extensive, and the walls had a distinctive honeycomb of large endodermal cells. In intermediate sized bracts ( Fig. 7 View FIGURE 7 B) the canal still retained thickened walls except towards its distal end. Proximally it extended slightly onto the upper side of the bract, while distally it ended below a relatively less prominent cluster of nematocysts. In the oldest bracts only the proximal part of the canal, in the region of attachment to the stem, remained slightly thickened and frequently it did not extend on to the upper side of the bract.
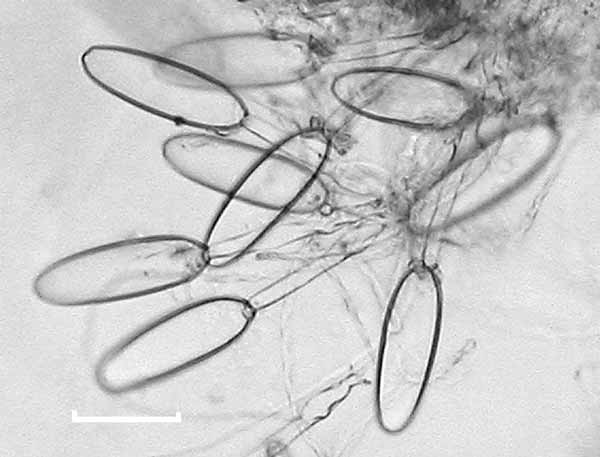
The cluster of nematocysts was always positioned at the distal tip of the bract. In the youngest bracts there were approximately 20–30 nematocysts, all of the same kind. In the material available, this number decreased as the bract increased in size, although it is not clear whether this decrease is truly an ontogenetic pattern or whether the losses occurred through abrasion or preservation. The nematocyst capsules ( Fig. 8 View FIGURE 8 ) measured 82 µm in length and 25 µm in width. The shaft measured 78 µm in length and had a few small barbs at its distal end. They are most probably microbasic mastigophores.
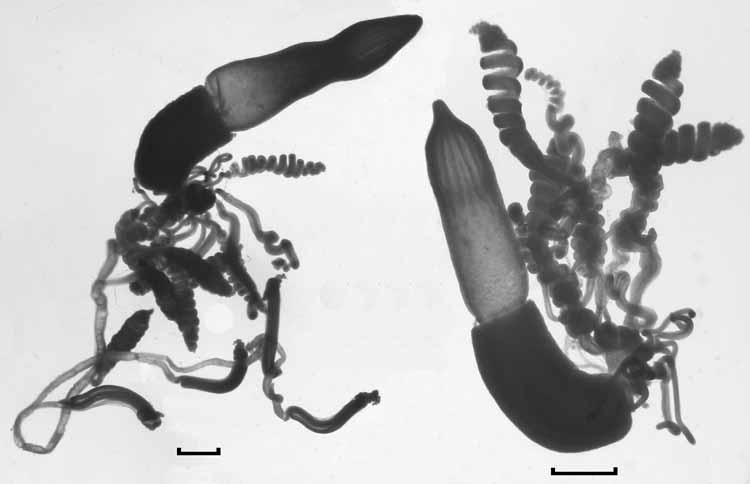
Palpon: The palpons ( Fig. 9) measured up to 8 mm in length and 2.7 mm in width, although the younger ones remained relatively narrow. Over 80 were found with the St. 67 specimen, together with about 12 gastrozooids, indicating that each cormidium bore at least six palpons. They were largely featureless, the younger ones often transparent, while the older ones were filled with a dense, granulose substance. The surface was covered in a dense array of patches of 6–12 ectodermal cells. There was a small proboscis at the distal end, and a constricted process at the proximal end from which the palpacle arose. The latter was long and narrow, without annulations, although at irregular intervals there were small constrictions. No nematocysts were discerned anywhere on the palpon or the palpacle.
Gastrozooid and tentacle: The gastrozooids ( Fig. 10 View FIGURE 10 ) were attached directly to the siphosomal stem, and measured up to 7.6 mm in length and 1.8 mm in diameter. Frequently each of the three main regions, the basigaster, stomach, and proboscis, occupied approximately one third of the total length of the gastrozooid, although the proboscis region could be very variable in shape. However, in one case ( Fig. 10 View FIGURE 10 , right) the basigaster was greatly enlarged and occupied almost half the length of the gastrozooid, but still was relatively narrow. Twelve so-called longitudinal liver stripes were present in the proboscis region.
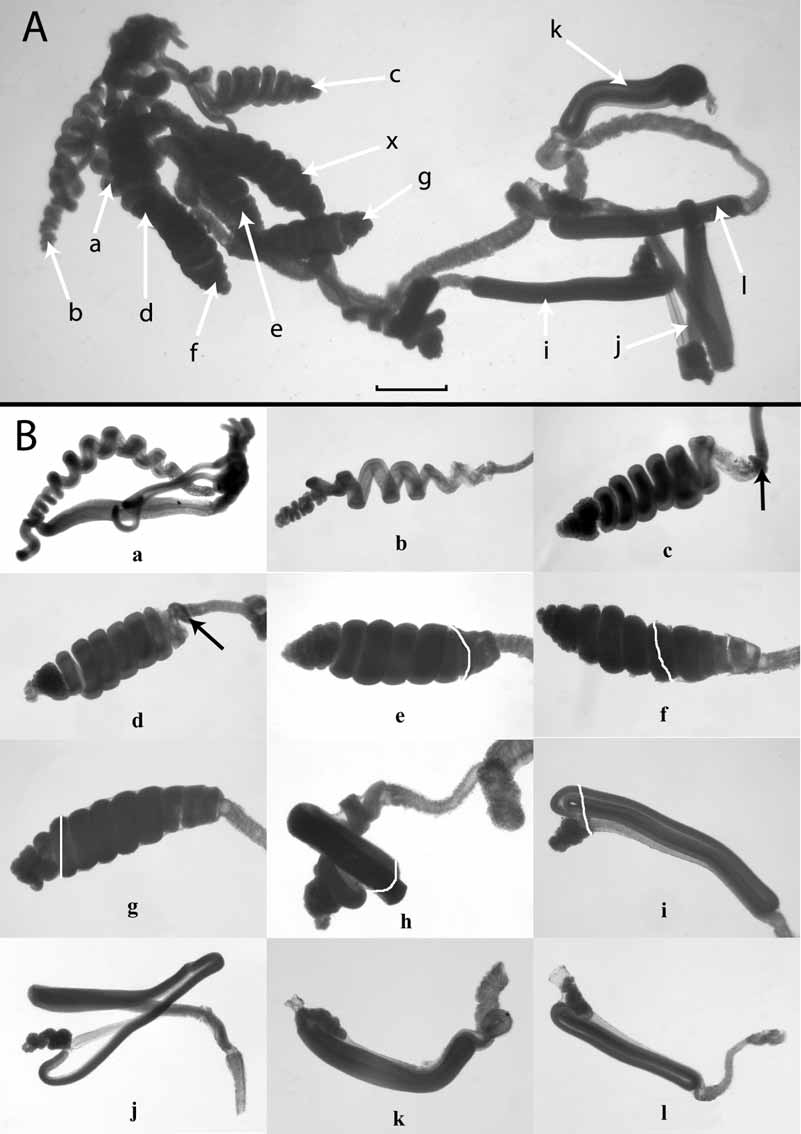
The annulated tentacle arose at the very base of the gastrozooid and bore two basic forms of tentilla ( Fig. 10 View FIGURE 10 , 11 View FIGURE 11 A), which arose, as usual, at the tentacular nodes. The youngest, proximal tentilla ( Fig. 11 View FIGURE 11 B a) were simple straight tubes that, as they enlarged, began to differentiate their three basic regions, the pedicle, cnidoband, and terminal filament. The cnidoband then began to coil up into a loose spiral ( Fig. 11 View FIGURE 11 B a, b). As this spiral tightened an involucrum began to develop at the very base of the cnidoband ( Fig. 11 View FIGURE 11 B c, d — arrowed). This involucrum gradually extended distally ( Fig. 11 View FIGURE 11 B e -g — the distal end of the involucrum is marked by a white line) so as to almost or entirely cover the now tightly coiled cnidoband. At this point of development of the tentilla something occurred that has not been previously described for any siphonophore. The cnidoband began to unwind ( Fig. 11 View FIGURE 11 B h) and completely change its topography such that it eventually arranged itself into three straight zigzagged sections ( Fig. 11 View FIGURE 11 B i). On this particular tentacle this transition apparently took place when the involucrum had almost, but not entirely, covered the cnidoband. During the transition parts of the cnidoband projected distally beyond the involucrum, and once the transition was complete the involucrum still did not entirely cover the now zigzagged cnidoband. The tentillum that was the third from the distal end of the tentacle ( Fig. 11 View FIGURE 11 B j) had lost its involucrum and clearly shows the overall arrangement of the three main zigzagged sections of the cnidoband, with the socalled “double elastic band” connecting its proximal and distal ends. The two distal most tentilla ( Fig. 11 View FIGURE 11 B k–l) show that the involucrum continued to extend distally and eventually covered the whole of the cnidoband and, indeed, extended well beyond it so that the terminal filament could be withdrawn entirely within it. The distal end of the cnidoband turned back proximally to form the beginnings of a fourth zag, and the terminal filament arose at its end.
Unfortunately, the tentacle illustrated in Fig. 11 View FIGURE 11 was the only one where a tentillum was found to be in the transitional stage between the more proximal ones with a coiled cnidoband and the more distal ones with a zigzagged cnidoband. In addition, it is uncertain whether the transition begins before or after the cnidoband is completely enveloped by the involucrum. The most advanced pre-transitional tentillum was found to have the involucrum covering all of the cnidoband, together with the base of the terminal filament. However, it is clear that a transition between the two forms of tentilla does occur, rather than a succession of the zigzagged type being developed first followed by a switch over to the development of the coiled type as the gastrozooid and tentacle mature. In that case one would expect the more anterior gastrozooids to have tentacles bearing only the zigzagged form. However, all of the gastrozooids, including one still attached toward the anterior end of the siphosome, bore tentacles with coiled tentilla at their proximal ends, and half of them had zigzagged tentilla distally. In addition, broken off pieces of tentacle generally bore only zigzagged tentilla.
Four types of nematocysts were found to be present on the tentilla. Haplonemes, probably homotrichous anisorhizas, and heteronemes (microbasic mastigophores) were present on the cnidoband, while what were presumed to be desmonemes and acrophores were found in the terminal filament, although no discharged ones were found. However, these last two types of nematocysts are quite typically found on the terminal filaments of physonect siphonophores. About 300 mastigophores, measuring 80–106 x 21 µm, werefound at the proximal end of the cnidoband, flanking the haplonemes. They were present on the first two or three spirals of the coiled cnidobands and along the first zag of the zigzagged cnidobands. Their density was greatest proximally and diminished distally, becoming widely spaced apart before they disappeared entirely. Innumerable haplonemes, generally of two sizes (53– 58 x 8 µm and 80– 85 x 10.5 µm), were present throughout the cnidoband and arranged into several rows. Both the presumed desmonemes ( 25–26 x 17.5–18.5 µm) and acrophores (24– 28 x 8–9.25 µm) varied in size, and the latter were far more abundant than the former but, because the terminal filaments were tightly coiled, their exact arrangement could not be elucidated.
Moser (1925) clearly illustrated the arrangement of the cnidoband in the zigzagged forms of tentilla (see Fig. 1 View FIGURE 1 ). She showed that the nematocyst-bearing side of the first zag lay against that of the second, while that of the third lay on the outer surface of the cnidoband. She also clearly showed the strong and doubled elastic band running up the exterior side of the first zag. She believed that a much reduced elastic band was present in the second and third zags, but this may not be the case. In the spiralled cnidobands the double elastic band clearly was present throughout the length of the cnidoband, but in the zigzagged ones it directly connected the proximal and distal ends of the cnidoband (see Fig. 11 View FIGURE 11 B j) and, thus, could not be present between the second and third zags.
Gonophores: Unfortunately, only the anterior part of the siphosome of both Polarstern specimens was preserved, and no ripe gonophores could be found. Groups of small, rounded buds were present, some of which were clearly destined to become female gonophores as the nucleus of each could clearly be seen. However, no buds of male ones could be discerned, although this does not necessarily mean that they were absent. However, as noted below, some of Totton’s material that has been re-examined clearly showed that both male and female gonophores were present on the same specimen.
Distribution: There are very few published records of Resomia convoluta , all from the Antarctic Ocean apart from an extremely unbelievable one from Baja California ( Alvariño 1991). Nonetheless, it is possible that this latter record may refer to another Resomia species that has yet to be described (Haddock and Pugh, in preparation). The original R. convoluta material came from Posadovsky Bay (c. 65°S, 89°E) from depths of 400 (2 records) and 3243 m, although it is likely that open nets were used. There were no further records until Totton (1965) mentioned that the species had been collected at two or three Discovery stations. However, the Resomia material presently in the Natural History Museum, London, all of which Totton had looked at, actually comes from at least eight Discovery stations. In addition, Totton believed that this material represented more than one Resomia species. These points are considered further in the Discussion.
More recently, records for nectophores of Resomia ( Moseria) sp. have been given by Margulis (1992), from two stations in the Commonwealth Sea (60+°S, 55–90°E; depth ranges 100–50 and 2000– 1000 m); Pagès et al. (1994) and Pugh et al. (1997) from various stations and depths in the Atlantic sector of the Subantarctic and Antarctic. Finally, Pagès and Kurbjeweit (1994) reported the collection of 20 colonies of R. (M.) contorta from various stations in a transect across the Weddell Sea, in the depth ranges 1000–500 and 500– 200 m.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SubOrder |
Physonectae |
|
Family |
|
|
Genus |