Baccharis heeringiana Malagarriga (1954: 6)
|
publication ID |
https://doi.org/10.11646/phytotaxa.632.2.5 |
|
DOI |
https://doi.org/10.5281/zenodo.10515500 |
|
persistent identifier |
https://treatment.plazi.org/id/03905372-FFD1-FFEA-FF4F-6392CDD5F944 |
|
treatment provided by |
Plazi |
|
scientific name |
Baccharis heeringiana Malagarriga (1954: 6) |
| status |
|
Baccharis heeringiana Malagarriga (1954: 6) View in CoL ,
Type:— BRAZIL. São Paulo, Campo Congonhas , pistillate, 23–27 March 1946, W.Hoehne 1948 ( lectotype RB barcode RB162551!, designated by Heiden (2009: 976) ;
isolectotypes ICN barcode ICN031862 !, SPF barcode SPF11948 !).
= Baccharis macroptera Hind (1993: 261) . Type:— BRAZIL. Bahia. Água Quente, Pico das Almas , Vertente Norte , vale ao noroeste do pico , 1500 m, beira de rio, 20 December 1988, R.Harley et al. 27311 ( holotype SPF barcode SPF 70298 !; isotypes CEPEC!; K barcodes K 000053093!, K 000053094, SP barcode SP 000505!, U barcode U 001210!, US barcode US 00432882!).
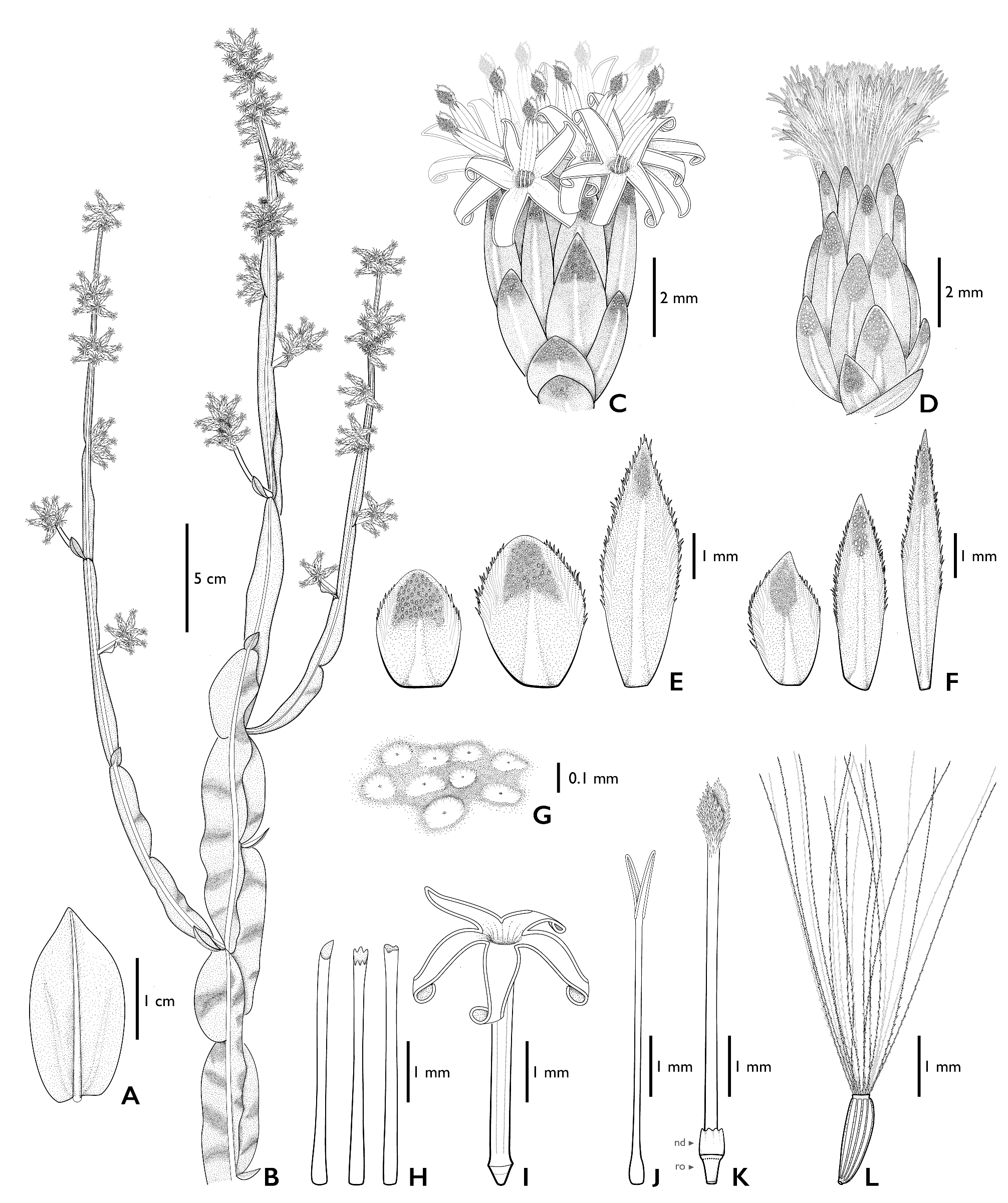
Subshrubs to shrubs 70–125 cm tall, stems erect, branching from the base, glabrescent, green to grayish-green, 3- winged, wings 0.6–16 × 0.1–2 (–2.5) cm, oblong, elliptical, to obovate, membranaceous to coriaceous, flat to somewhat wavy. Fertile stems with wings remarkably narrowed towards the apex. Leaves alternate, spiral, sessile, gradually reduced towards the apex, the basal ones 1.35–2.25 × (0.35–) 0.6–0.9 cm, elliptic, narrowly elliptic to obovate, base truncate, rounded or sagittate, apex acute-acuminate, glabrous, 1-veined and with two somewhat conspicuous lateral veins towards the base, margins entire; the apical ones (0.3–) 0.5–1 × (0.1–) 0.25–0.4 cm, narrowly elliptic to narrowly ovate, truncate base, acute-acuminate apex, glabrous, 1-veined and with two slightly conspicuous lateral veins, margins entire. Capitula sessile, in clusters of 2–9 capitula; clusters arranged in pseudo-spikes (7–) 9.5–18 (–25) cm long, in terminal and lateral branches. Staminate capitula with involucres 5.5–7 (–9) × 3–4.5 (–6.5) mm, cylindrical, phyllaries in 5–6 series; outer phyllaries 2.3–2.5 × 1.3–1.5 mm, ovate, base truncated, apex acute, resinous, puberulous near to central vein, margins entire to ciliate, hyaline-membranaceous; inner phyllaries 5.3–5.5 × 1–1.2 mm, narrowly elliptical, base truncated, apex acute, puberulous near to central vein in the apical third, margins hyaline-membranaceous, ciliate in the apical half. Receptacles flat, areolate, surface between areoles covered by glandular trichomes. Florets (20–) 30–41, corollas tubular, white, 5-lobed, tubes 3.8–5.5 mm long, cylindrical with a uniform width, throat absent or up to 1 mm long, lobes 1.5–2.2 mm long, narrowly elliptical, apex acute, glabrous except for very few short simple eglandular trichomes towards apex; anthers 1.1–1.5 mm long, oblong, base rounded, connective appendix 0.4–0.5 × 0.15–0.2 mm, ovate, apex acute; style 5–7.7 mm long, styles branches 0.8–1 mm long, fused or free, obovate, apex acute, collector trichomes on the abaxial face, stylopodium absent, nectary disc 0.5 mm high, ovary 0.3–1 mm long, rudimentary. Pappus 5.5–6.5 mm long, bristles 19–21, scabrid, frizzy, barbellate, and slightly flattened towards the apex, fused at the base, 1-seriate. Pistillate capitula with involucres 6–7.5 (–9) × 3–5 mm, cylindrical to ovoid, phyllaries in 5–7 series; outer phyllaries 2.2–2.5 × 1.5–2 mm, ovate, base truncated, apex acute, puberulous, resinous and glandular, margins hyaline-membranaceous, entire at the base and ciliate at the apex; inner phyllaries 5.3–6 × 0.7–0.8 mm, narrowly elliptical to linear, base truncated, apex acute, puberulous on the apex near to central vein, margins hyaline-membranaceous, entire at the base and ciliate at the apex. Receptacles flat to slightly convex, areolate, surface between areoles covered by glandular trichomes. Florets 55–157, corollas filiform, 3–5 mm long, white, 3–5-lobed, lobes obtuse unequal, sometimes with a short limb 0.2–0.3 mm long; styles 3.5–5.5 mm long, style branches 0.7–1 mm long, triangular to narrowly elliptic, apex acute attenuate. Cypsela (0.8–) 1–1.5 mm long, fusiform, terete, 6–9 ribbed, ribs covered with papillae, carpopodium present. Pappus (3–) 4–5.5 mm long, bristles 18–20, barbellate at the base and slightly scabrid towards the apex, fused at the base, 1-seriate, persistent ( Figs. 1–3 View FIGURE 1 View FIGURE 2 View FIGURE 3 ).
Habitat:— Baccharis heeringiana occurs in a very specific habitat, growing in vegetation types developed on waterlogged hydromorphic soils. The species commonly occurs associated with Eryngium pandanifolium , Leptostelma tweediei and Cyperus byssaceus .
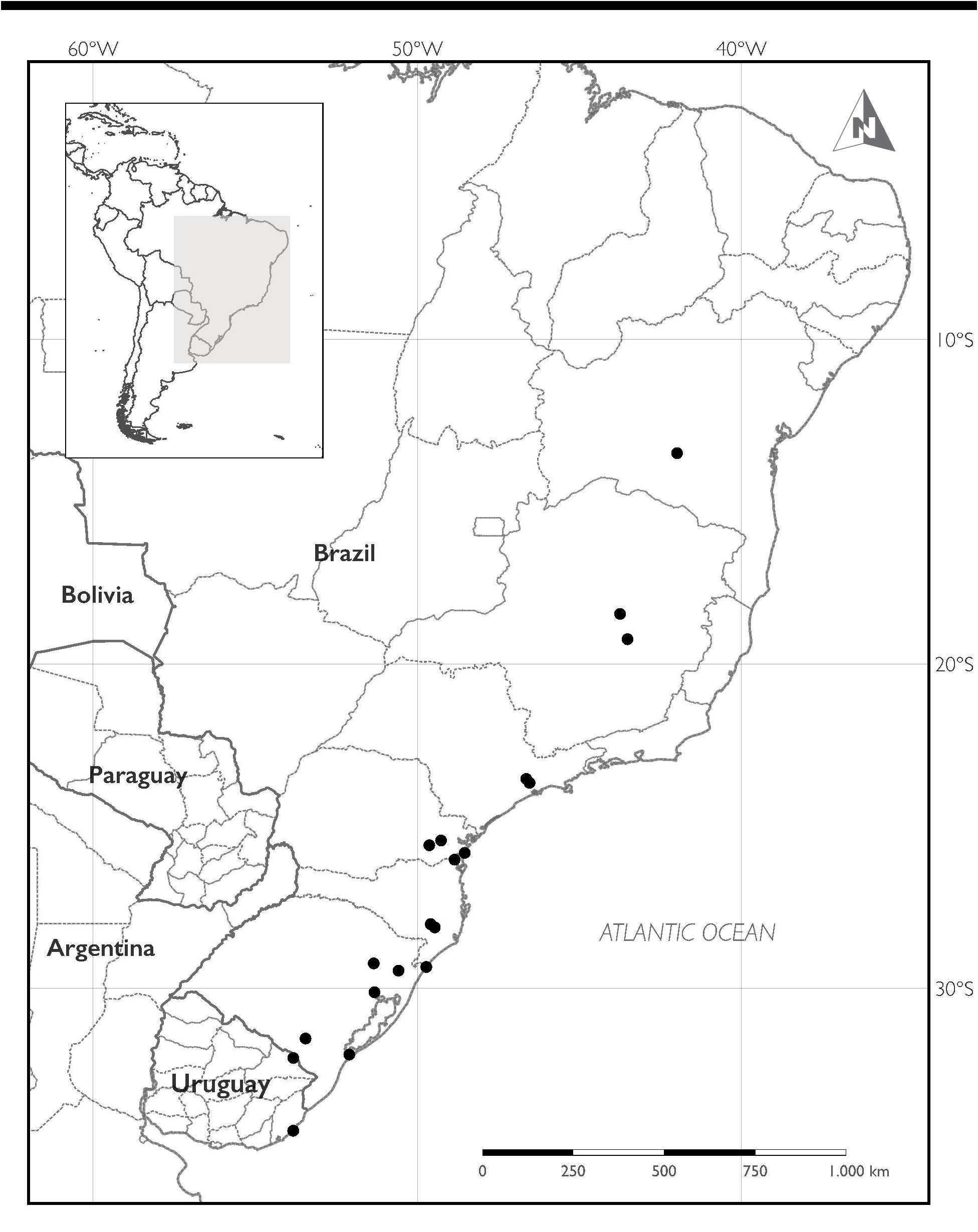
Distribution:— Baccharis heeringiana is widespread with a scattered distribution throughout eastern Brazil, occurring from north to south and from inland towards the coast in the states of Bahia, Minas Gerais, São Paulo, Paraná, Santa Catarina, and Rio Grande do Sul. In Uruguay, it is here reported for the first time from the departments of Cerro Largo and Rocha ( Fig. 4 View FIGURE 4 ).
Phenology:—Flowering from December to March, fruiting from February with fruit dispersion until July.
Conservation assessment:— Baccharis heeringiana was categorized as Endangered ( EN) in the AOO analysis. The analysis with the GeoCAT tool (Bachmann et al. 2011) estimated an area of occupancy ( AOO) of 80 km 2; based on the IUCN guidelines (2019), it is likely that this species would probably be classified as Endangered ( EN B 2a+ B 2b) if subjected to a formal assessment. As we mentioned above, although B. heeringiana has a wide geographic distribution, its habitat is fragmented and very specific, consisting of sites with waterlogged and hydromorphic soils where even minor alterations in the drainage conditions could considerably impact the conservation of its populations.
Taxonomic history:— Malagarriga (1954) described Baccharis heeringiana based on specimens collected by Hoehne in São Paulo, Brazil. Interestingly, Malagarriga described the species as a hybrid using morphological characters of the leaves and wings to explain the proposed “hybrid origin”, which he postulated resulted from the cross between B. usterii Heering (1911: 260) (= B. junciformis Candolle (1836: 426)) and B. milleflora ( Lessing 1831: 143) Candolle (1836: 426) .
In the treatment of the subtribe Baccharidinae Lessing (1830: 145) from Brazil, Barroso (1976) considered B. heeringiana as a synonym of B. usterii . In that same treatment, Barroso also included B. heeringiana in a list of doubtful species. Later, in the treatment of the tribe Baccharidinae for the Flora Ilustrada Catarinense, Barroso & Bueno (2002) accepted the synonymy proposed by Barroso (1976) without mentioning its doubtful status.
Schneider et al. (2009) recognized B. heeringiana as a separate species and removed it from the synonymy of B. usterii . They considered that there were enough morphological differences to recognize both taxa, and they also did not find enough arguments to justify the recognition of B. heeringiana as a hybrid as proposed by Malagarriga (1954). Additionally, in that same contribution, they added B. macroptera Hind (1993: 261) to the synonymy of B. heeringiana because of shared morphological characters, the overlap of both species’ diagnostic characters, and the analysis of the type specimens. A few months later, in their taxonomic study of B. sect. Caulopterae from Rio Grande do Sul, Heiden et al. (2009) reversed their earlier decision, including B. heeringiana in the list of synonyms of yet another species, B. sagittalis , which was followed by Müller (2013).
Note 1: —The taxonomy presented here recognizes B. heeringiana as a distinct species, excluding it from the synonymy of B. sagittalis . This is based on a detailed study of the morphology of B. heeringiana across its geographic range as well as an analysis of the protologues and type material.
Baccharis heeringiana has wider wings than B. sagittalis ( 6.5–20 mm vs. 2–6 mm wide), longer corollas in staminate florets (5.5–8 vs. 3.5–4.5 mm long), longer styles in pistillate florets (4–6.5 vs. 2–2.6 mm long) and different pappus elements (barbellate vs. scabrid bristles) (see Table 1 View TABLE 1 ).
Baccharis saggitalis s.l. as circumscribed by Müller (2006, 2013) represents a species complex that requires further studies at morphological and molecular levels to further clarify its status as a single polymorphic entity or of a group of related taxa, as hitherto this taxon has not been included in a phylogenetic analysis.
Note 2: —In 1954 Malagarriga described Baccharis heeringiana as a hybrid taxon, resulting from the putative cross between B. usterii (= B. junciformis ) and B. milleflora . He used morphological characters of the leaves and wings to explain the possible hybrid origin. We believe there exists enough morphological, geographical, and molecular evidence to support the identity of the species as a separate taxon with an independent origin, especially because B. heeringiana has stable populations as observed in our fieldwork and its distribution rarely overlaps with those of B. junciformis and B. milleflora , which also are partially overlapping only in few localities in the states of São Paulo, Paraná, Santa Catarina and Rio Grande do Sul in Brazil. Moreover, B. millefora is not recorded to Bahia, in Brazil, and neither in Uruguay.
Although B. heeringiana , B. junciformis , and B. milleflora belong to B. sect. Aphyllae and present some similarities such as their subshrubby to shrubby habit, 3-winged stems, capitula arranged in clusters, and similar habitats, B. heeringiana differs from the other two species by several character states detailed below.
Baccharis heeringiana differs from B. junciformis in its shorter leaves (1.35–2 vs. 1.5–6 cm long), capitulescence arrangement (pseudo-spike vs. paniculiform), longer staminate involucres (5.5–7 (–9) vs. 4–5.5 mm long), longer pistillate involucres (6–7.5 (–9) vs. 5–6.5 mm long) and longer staminate corollas (5.5–8 vs. 3.5–5 mm long). These species also differ in their flowering period, as B. heeringiana flowers from December to March and B. junciformis from February to June. Baccharis heeringiana differs from B. milleflora in its capitulescence arrangement (pseudo-spike vs. paniculiform), longer staminate involucres (5.5–7(–9) vs. 3–4 mm long), longer pistillate involucres (6–7.5 (–9) vs. 4–5.5 mm long), pistillate capitula with a greater number of florets (55–157 vs. 40–52), longer staminate corollas (5.5–8 vs. 2.3–2.8 mm long), longer pistillate corollas (3–5 vs. 1.8–2.2 mm long) and longer pistillate styles (4–6.5 vs. 2.2–3.2 mm long).
In addition to the morphological characters presented here, the molecular evidence presented by Heiden et al. (2019) does not support the hypothesis of B. heeringiana ’s hybrid origin. Heiden et al. (2019) based on molecular phylogenetic reconstruction demonstrated that the clade of B. sect. Aphyllae (pp=1) split into two clades, one clade (pp=1) where B. heeringiana (as B. sagittalis — voucher specimen Heiden et al. 1329) is sister to the lowland Pampean B. phyteumoides ( Candolle 1836: 425) and the two are sister to a clade (pp=0.98) containing B. junciformis and additional seven species mostly occurring in the Atlantic Rainforest domain and its associated high elevation grasslands. Meanwhile, B. milleflora is placed in a low supported clade (pp=0.57) containing 18 species with distributions scattered in South America, where it is in a clade (pp=1) of 10 exclusively Atlantic Rainforest domain species, being sister (pp=1) to nine species mainly from the high elevation grasslands. This hypothesis showing B. heeringiana as phylogenetically closer to B. junciformis and far from B. milleflora is not in accordance with a likely hybrid origin from a crossing between B. junciformis and B. milleflora . Moreover, Bayesian inference tree based on the nuclear subset analyses ( ETS + ITS) recovered similar relationships as the total evidence analysis. The Bayesian inference tree based on the plastid subset analyses ( trn H- psb A + trn L-F) did not have enough resolution to infer relationships other than B. heeringiana as sister of B. phyteumoides and both sister of a clade containing B. junciformis as the earliest divergent lineage of a clade containing seven species mainly from the Atlantic Rainforest high elevation grasslands, while B. milleflora emerged as sister of B. flexuosiramosa Schneider & Boldrini (2008: 48) , both positioned in a large polytomy with random species from diverse subgenera.
Additional specimens examined:— BRAZIL. Bahia: Água Quente; Pico das Almas ; -13.5, -41.983333; 20 December 1988, R.M. Harley et al. 27310 ( MBM 151333 !) . Minas Gerais: Jaboticatubas, Serra do Cipó, Área de Proteção Ambiental Morro da Pedreira, rodovia Belo Horizonte — Conceição do Mato Dentro ( MG 010), km 129 atual, margens da rodovia; S 19º 14’ 2”, W 43º 30’ 37”; 18 February 2011, G. Heiden et al. 1621 ( RB 597232!, MVFA!*) GoogleMaps . Paraná: Matinhos, Litoral Paranaense ; -25.8174991607666, -48.5428009033203; 08 March 2003, J.M. Budel s.n. ( ICN 127131 !) . Rio Grande do Sul: Pinheiro Machado, Torrinhas , Rodovia BR 293 , km 114; S 31° 32’ 26’’, W 53° 26’ 57’’; 27 October 2012, G. Heiden et al. 2016b ( MVFA!*) GoogleMaps . Guaíba ; -30.113899230957, -51.3250007629395; 13 April 2008, N. I. Matzenbacher s.n. ( ICN 153618 !) . Farroupilha, 7 km antes de Caxias do Sul; -29.2250003814697, -51.3478012084961; 03 January 1994, L.T. Pereira 62 ( ICN 119270 !) . Santa Catarina: Urubici, Parque Nacional de São Joaquim, Morro da Igreja ; S 28º 7’ 0”, W “49º 28’ 0”; 02 April 2010; G. Heiden et. al 1329 [as B. sagittalis in the phylogeny by Heiden et al. 2019] ( RB 572455!) . São Paulo: Cidade Jardim ; -23.5400009155273, -46.6300010681152; 20 March 1946; W. Hoehne 1953 ( ICN 31859 !) . Congonhas , 27 March 1946; W. Hoehne 11948 ( MBM 190229 ) .
URUGUAY. Cerro Largo: Sierra de Ríos; S 32° 08’ 29.2’’, W 53° 49’ 29.6’’; 13 December 2016, C. Trujillo et al. 17 ( MVFA!*) GoogleMaps ; 13 December 2016, C. Trujillo et al. 18 ( MVFA!*) ; 15 March 2017, C. Pérez et al. 26 ( MVFA!) . Rocha: Parque Nacional Cabo Polonio, S 34° 22’ 56.9’’, W 53° 49’ 45.8’’, 23 February 2016, V. Valtierra et al. 70 ( MVFA!*) GoogleMaps ; 23 February 2016, V. Valtierra et al. 74 ( MVFA!*) ; 8 December 2016, V. Valtierra et al. 121 ( MVFA!*) ; 8 December 2016, V. Valtierra et al. 122 ( MVFA!*) .
Specimens examined of Baccharis sagittalis : URUGUAY. Florida: Arroyo Timote; Estancia Santa Clara ; 4 December 1938, B. Rosengurtt PE 3733 ( MVFA!) . Rincón de Santa Elena ; 11 February 1946, A. Lombardo 3726 ( MVJB 10183 !) . Maldonado: Pan de Azúcar; 19 January 1913, C. Osten 6478 ( MVM!) . Montevideo: Cerca de los Arroyos ; November 1874, J. Arechavaleta 4089 ( MVM!) . Rocha: Sierra de San Miguel; 3 November 1874, G. W. Teague ( MVM 15185 !) . Salto: Salto Grande entre Aº Espinillar y Río Arapey ; S 30º 57’ 41.86”, W 57° 52’ 5.58”; 27 November 1977, O. Del Puerto ( MVFA 14561 !) GoogleMaps . San José: Río Santa Lucía ; 25 November 1929, C. Osten 21723 ( MVM!) . Predio Forestal Arazatí ; S 34° 32’ 4.3”, W 57° 01’ 10.9”; 2 December 2016, V. Valtierra et al. 118 ( MVFA) GoogleMaps ; 2 December 2016, V. Valtierra et al. 119 ( MVFA) . Soriano: Arroyo Grande, Paso de los Loros , 24 November 1940, B. Rosengurtt PE 4491 ( MVFA) .
| ICN |
Colombia, Bogota, Universidad Nacional de Colombia, Insituto de Ciencias Naturales de la Universidad Nacional |
| MVFA |
MVFA |
| MVM |
MVM |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |