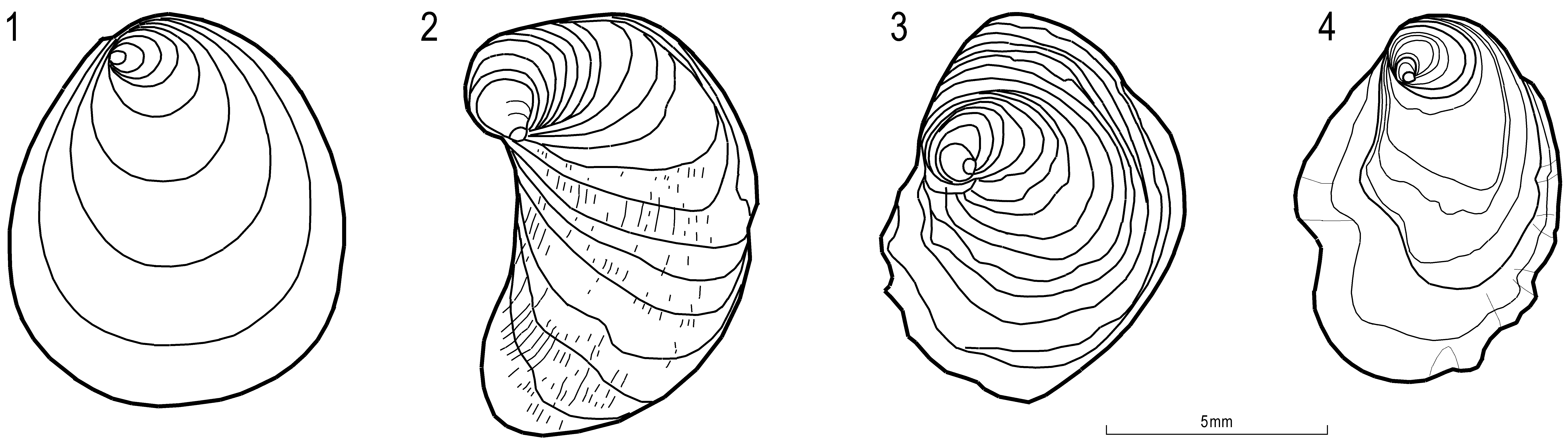
Circunula, Koppka, Jens, 2015
|
publication ID |
https://doi.org/10.11646/zootaxa.3927.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:42B56D11-9B18-4FCC-B632-30A46AB0205C |
|
DOI |
https://doi.org/10.5281/zenodo.6102686 |
|
persistent identifier |
https://treatment.plazi.org/id/039087D7-C015-462E-FF68-FC36FABF36BC |
|
treatment provided by |
Plazi |
|
scientific name |
Circunula |
| status |
|
Classification outline
Molecular phylogenetics seems to be the only method capable of providing some invariant anchor points to oyster systematics which necessarily needs to be interwoven with morphological data from both living and fossil taxa. Hence, the starting point for the current classification scheme is based on the most comprehensive molecular analyses currently available and complemented by the paleontological approach of Carter et al. (2011) (see Bieler et al. 2010 for an alternative view).
According to the genetic data of Giribet & Distel (2003, fig. 3.5) and Ó Foighil & Taylor (2000, figs. 2–5), Lophinae oysters appear either as a sister taxon to, or some species also nested within, Ostreinae rather than being basal to them. Hence, the two subfamilies form a crown group which is here synonymized with the Family Ostreidae .
Crassostreinae essentially form a sister taxon to this “restricted” family Ostreidae , and either a Saccostrea or a Crassostrea species appears at the base. Consistent in all analyses, Recent Gryphaeidae form the sister taxon of Ostreidae plus Crassostreinae , and the superfamily of Recent Ostreoidea appears very robustly as a monophylum: Gryphaeidae ( Crassostreinae ( Ostreinae , Lophinae ).
In the present context, these genetic results suggest that Mesozoic Lopha -like oysters, here represented by the Jurassic genus Actinostreon , are convergent to Recent Lophinae justifying the placement of these Mesozoic taxa outside Lophinae and outside Ostreidae , that is, in Arctostreidae Vialov, 1983 (Carter et al. 2011, p. 8; Malchus 1990) (see Hautmann 2001, for a contrasting view). In addition, as a consequence of excluding Crassostreinae from Ostreidae , this subfamily is included in the Family Flemingostreidae Stenzel, 1971 (Carter et al. 2011).
Indirectly, the genetic results also suggest that brooding evolved only once in Recent oysters, converting this character in a potential autapomorphy of the stem species of Ostreidae (see Ó Foighil & Taylor 2000, for anatomical arguments). The time when this happened cannot be ultimately fixed. However, current evidence from fossil larval shells hints towards the Tertiary (Eocene, Miocene) ( Malchus & Sartori 2013, p. 78, 84, 86). All pertinent results from the present study are consistent with this view [e.g., Pl. 9.10c–e, Pl. 10.1–2 ( Nanogyra ), Pl. 6.5, 10.3a ( Praeexogyra ), Pl. 19.4a–d ( Actinostreon )]. As of today, it appears therefore likely that none of the Jurassic Ostrea- or Crassostrea -like taxa belongs to the Ostreidae as defined above.
Species LV outline LV convexity LV sculpture RV convexity RV sculpture Resilifer Posterior Chomata Microstructure
adductor scar
Circunula n. gen. round low, ventral margin dorsally with weakly faint radial curved round only in SP, RF, CCF cotyledon upturned radial furrows inflated threads juveniles Nanogyra ( N.) oval capacious smooth convex- commarginal exogyrate dorsally absent SP, RF nana concave growth crests biconcave
Nanogyra ( P.) kidney weakly inflated smooth flat, weakly smooth, faint exogyrate round present RF, lenses? reniformis convex radial threads
Nanogyra ( P.) comma capacious numerous radial convex- faint radial exogyrate oval present RF, lamellar virgula riblets concave threads lenses Praeexogyra drop capacious commarginal flat, ventrally ventrally faint trigonal, dorsally absent SP, RF dubiensis growth lines, concave radial threads oblique biconcave
weak swellings
Praeexogyra kidney capacious commarginal flat, ventrally faint radial trigonal, high-oval absent RF, CF monsbeliardensis swellings concave threads oblique
Helvetostrea n. trapezoid, capacious, thick irregular growth flat unknown ostreoid, high-oval absent RF, CCF, large gen. sequana tube-like shelled squamae massive chambers
bourrelets
Actinostreon broadly convex plicae (5–35) convex plicae (5–35) weakly round, present RF, CCF, greagareum crescentic convex dorsally flat to hollow (RV), weakly chambers (both massive concave valves) bourrelets
These "cornerstones" apart, the evolution of Jurassic oysters, especially the phylogenetic relationships between Circunula n. gen, Helvetostrea n. gen., Praeexogyra , and Nanogyra described herein and the true origin of Liostrea , Catinula , Praeexogyra , and Crassostrea remain a puzzle. Therefore, the presently proposed classification is necessarily tentative.
Important morphological features. Classical characters examined in this approach are shell shape, external ornament, phenotypic variation, coiling, muscle-scar shape and position, chomata, microstructure (e.g. Aqrabawi 1993; Carter & Malchus in Carter et al. 2011; Hautmann 2001; Malchus 1990, 1998; Stenzel 1971), and as far as possible the larval shell ( Malchus 1995, 2000, 2004a; Malchus & Sartori 2013). For comparison of characteristic shell features of the Reuchenette oysters see Table 1.
It was found, however, that many of the postlarval characters, which are generally accepted as diagnostic at various taxonomic levels, can vary with age (size). Although strongly limited by taphonomy and diagenesis, the present approach thus tried to include even the smallest specimens of each species to capture information on their earliest shell stage characters. Some relevant examples are briefly circumscribed:
Coiling—Early postlarval growth in oysters is almost intrinsically anterior-directed helicoidal thus producing an opisthogyrate, or at least opisthocline umbo. This appears to be due to a delayed offset of the larval coiling tendency ( Malchus 2000, p. 308, text-figs. 1–4; 2004a, p. 99, characters E to I; 2004b, p. 1546, text-figs. 3b, 8). However, offset and even radical changes in coiling direction may occur very early after metamorphosis. This is, for example, the case in an unusually large number of individuals of Circunula n. gen. cotyledon which are prosogyrate in early postlarval life. Another example is Actinostreon gregareum which is actually strongly exogyroid in earliest postlarval life (Pl. 16.6; Pl. 18.1b,c; Pl. 19.4a–d). But this potentially important feature is rarely preserved because the juvenile umbo becomes easily eroded. Generally speaking, later growth phases are mostly characterized by gradual changes in the degree of coiling rather than by radical re-orientation. Gradual changes may also be diagnostic, as discussed here for various species of Nanogyra . Coiling and changes of coiling direction also affect the orientation and width of the ligament area which are thus not independent diagnostic features ( Malchus 2000, 2004a, b). These characters are often better visible in the free right valve ( Fig. 9 View FIGURE 9 ) than in the attached and often xenomorphic left valve.
Chomata—Malchus (1998) already mentioned that these denticle-like shell margin features of still unknown provenience ( Malchus & Sartori 2013) may disappear during ontogeny. It is again Circunula n. gen. cotyledon providing a case in point where the character is deemed to be species (or genus) specific. Chomata also seem to leave antimarginal riblets on the shell exterior, at least in Nanogyra ( Palaeogyra) . Hence, the presence/absence of an apparently independent external ornamental feature may actually be linked to the onset/offset of an internal shell feature.
Posterior adductor scar—Juvenile imprints may have a different shape, orientation and position compared with their adult pendants. The posterior adductor of juvenile Actinostreon gregareum , for instance, leaves an “elongated” imprint (Pl. 18.3b) whereas that of the adult is round (Pl. 16.5b, 6a; Pl. 17.1b,c, 3b,c). In addition, the posterior border of the juvenile imprint corresponds to the ventral side of the adult adductor scar. It appears plausible to assume that this ontogenetic change largely corresponds to a rotational growth of the soft parts.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.