Branchinella hearnii, Timms, Brian V, 2012
|
publication ID |
https://doi.org/10.5281/zenodo.254592 |
|
DOI |
https://doi.org/10.5281/zenodo.5685429 |
|
persistent identifier |
https://treatment.plazi.org/id/03909832-FFEE-0015-6CC0-FB87D746FA97 |
|
treatment provided by |
Plazi |
|
scientific name |
Branchinella hearnii |
| status |
sp. nov. |
Branchinella hearnii View in CoL sp. nov.
Figs. 1 View FIGURE 1 F, 5B, 7.
Etymology. This species is named for Roger Hearn of Manjimup, who first collected this species from Little Unicup Lake, near Lake Muir in southwest Western Australia in 2005 and then displayed beautiful live specimens to the staff and myself as a visiting scientist at the Department of Environment and Conservation’s aquatic laboratory at Wanneroo.
Type locality. Western Australia, ca. 12 km N of Lake Muir, Little Unicup Lake, 34o 19’ 46.9”S, 116o 42’ 51.8”E, 19 August 2009, BVT
Holotype. Male deposited in the Western Australian Museum. Total length: 28 mm Accession number: WAM C49896.
Allotype. Female. Total length: 28 mm. Accession number: WAM C49897.
Paratypes. 1male, 5 females same data, WAM C49898; 2 male, 7 females, Western Australia, ca. 10 km N of Lake Muir and 1 km S of Unicup Lake, ‘Branchinella’ Lake, 34o 21’ 41”S, 116o 43’ 22”E, 6 October 2008, BVT, WAM C49899.
Other Material. Numerous juveniles, Western Australia, ca. 12 km N of Lake Muir, Little Unicup Lake, 34o 19’ 46.9”S, 116o 42’ 51.8”E, 5 September 2007, R. Hearn, WAM C49900; 1 male 9 females, Western Australia, ca. 14 km N of Lake Muir, Tolkerup West Lake, 34o 19’ 59.4”S, 116o 43’ 21.1”E, 6 September 2007, R. Hearn, WAM C49901; 1male, 1female, Western Australia, ca. 10 km N of Lake Muir and 1 km S of Unicup Lake, ‘Branchinella’ Lake, 34o 21’ 41”S, 116o 43’ 22”E, 2 November 2007, R. Hearn, WAM C49902; 12 juveniles, Western Australia, ca 18 km N of Lake Muir, ‘Cricket Ground Lake,’ 34o 16’ 17.5”S, 116o 42’ 03.8”E, 4 September 2008, R. Hearn, WAM C49903; 2 males, 4 females, Western Australia, ca. 14 km N of Lake Muir, Tolkerup West Lake, 34o 19’ 59.4”S, 116o 43’ 21.1”E, 6 October 1908, BVT, WAM C49904; many juveniles, 2.4 km SW of Watheroo, ‘Bentonite Lake,’ 30o 18’ 46” S, 116o 02’ 33”E, 5 September 2009, BVT, WAM C49905; 6 males, 2 females, 4.7 km W of Coomberdale, 30o 28’ 02”S, 115o 59’ 18”E, 6 September, 2009, BVT, WAM C49906.
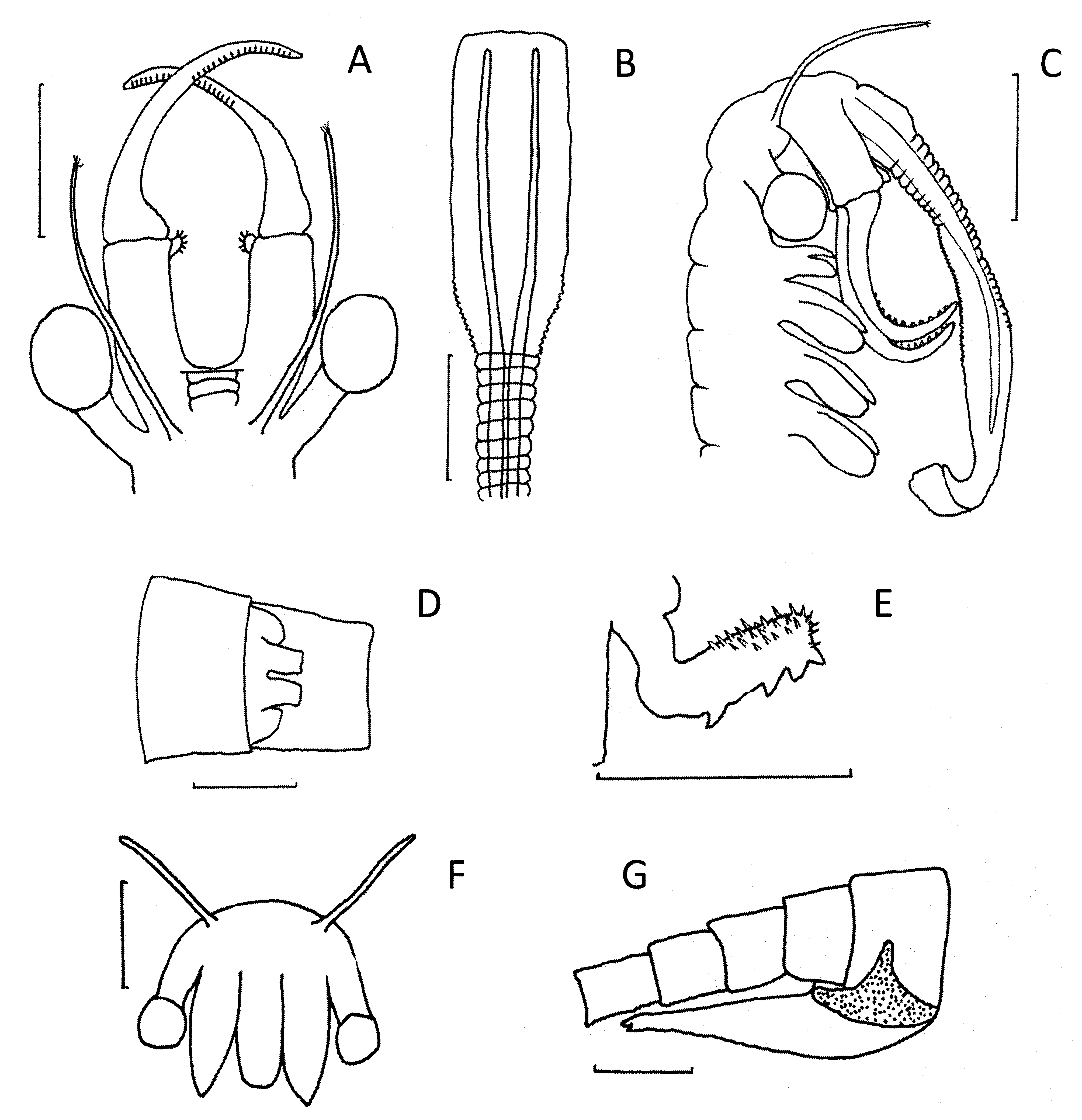
Diagnosis. Frontal appendage short and forked and not reaching beyond the bases of the second antennae. Second antenna medial surface of proximal antennomere with large plate like tumidity on each side. Each gonopod flanked by a lateral swelling with a central hollow. Females with a lateral tumidity on each genital segment and paired dorsal tumidities on the first genital segment. Anterior setae of fifth thoracopod with endites 4 and 5 anterior setae naked and more than twice as long as second anterior setae on endites 4–6 which carry a pecten of fine spines.
Description. Male. Eyes freely projecting on peduncles about the same length as eye diameter.
First antennae filiform, a little longer than second antenna proximal antennomere, and terminating in 3–5 long sensory setae.
Second antennae. Proximal antennomere slightly shorter than distal antennomere. Proximal antennomeres usually well separated from each other and fused basally at about 45o. Each proximal antennomere with a large lamellar projection aligned ventrally. This projection, lacking sensory denticles or spinules, supported posteriorly by a ventrally narrowing ridge. Distal antennomeres of uniform diameter, gently arcing medially, and with weakly developed transverse medial ridges.
Frontal appendage small, bifurcated, not protruding beyond the fused basal antennomeres.
Fifth thoracopod with endite 1+2and 3 evenly curved, the former about three times the size of the later. Anterior setae of endite 1 naked, of similar length to adjacent posterior setae. Anterior setae of endite 2 a little shorter than first anterior setae, bearing a pecten of spines and attended at its base by a small smooth spine. Endite 3 with an anterior setae longest of the three anterior setae on endites 1 to 3, about 7/8th the length of the adjacent posterior setae, and bearing a pecten of spines. This anterior setae also attended by a small spine at its base. Endites 4–6 asymmetrical and covered with small spines. Endites 4 and 5 with two anterior setae and endite 6 with one anterior seta, representing two types. The first of all three endites, about 1/3rd the length of the adjacent anterior setae and with a fine pecten most of their length. The second type on endites 4 and 5 only, 3/4 the length of adjacent posterior setae and naked. Posterior setae of all endites long and numbering about 27 on endites 1+2, about 9 on endite 3, then 4, 3, 4 respectively on endites 4–6. Those on endites 1+2 increasing in length basally to apex. Endopod broadly triangular shaped but with two closely spaced apices; straightest medial surface bearing about 12 spaced setae and curved lateral surface bearing numerous (ca 70) closely spaced setae. Exopod unevenly oval (medial edge more rounded than lateral edge) bearing about 90 closely spaced posterior setae. Setal bases with 3–6 minute spines. Epipodite elongate oval and unadorned. Praeepipodite large and broad, larger than endopod and exopod combined, and with a finely serrated margin.
Genital segments about same width as adjacent thoracic and abdominal segments. Bases of gonopods a little longer than one abdominal segment each flanked by a lateral swelling with a central hollow. Everted gonopod with a long row of about 15 triangular spines medially and a wide field of longer thin spines on opposite side.
Cercopods typical for Branchinella .
Female. Eye plus peduncle about half the width of the head.
First antenna filiform, a little longer than eye plus peduncle.
Second antenna lamellar, about three times the head width on each side and narrowing to a sharp symmetrical apex. Second antennae fused together medially.
Genital segments larger than adjacent thoracic and abdominal segments and with distinctive swellings and projections First genital segment with a large bulbous lateral tumidity each side and two adjacent asymmetrical pyramid shaped projections dorsally. Apices of these projections produced posteriorly and laterally. Second genital segment with another lateral tumidity on each side but it is sac-like and directed dorsally.
Brood pouch bulbous anteriorly with a marked ventral swelling, but tubular posteriorly and terminating at about the third abdominal segment.
Fifth thoracopod and cercopods as in male.
Egg diameter 485 µm, with about 13 polygonal depressions, each shallow with sharp ridges and concave floor with dimples (Timms and Lindsay, 2011).
Variability. Some variability in features was noticed in the material available. Fusion of the second antennal proximal antennomeres ranges from almost 90o to about 50o. In males the two ventral tumidities may be up to 25% smaller than in the holotype and the triangular spines on the gonopod may number up to 24 (see Figs 2 View FIGURE 2 A, B, Timms, 2008). In females the lateral tumidities on the genital segments may be smaller than in the allotype and the apices of the second antennae asymmetrical (see Figs 2 View FIGURE 2 C, D, Timms, 2008), and there may be two weak ventral hooks pointing posteriorly on the brood chamber. The paired dorsal pyramid-shaped projections are invariable.
Size. Mature B. hearnii sp.nov. are relatively large. There are 10 males in collections from the Lake Muir area ranging in size from 24 to 30 mm and averaging 26.7 mm, and 13 females in the same collections ranging in size from 19 to 38 mm and averaging 34.6 mm. Generally it is the smaller (immature?) males and females with the smaller tumidities.
Differential diagnosis. Branchinella hearnii sp. nov. is almost identical with B. compacta Linder , with which it was initially confused ( Timms, 2008), but its 16SmtDNA differs by 12.8% ( Pinceel et al., 2012). It is separable from this species by many morphological features, including: (a) in males, the ventral lamellar projection on the second antennal proximal antennomere is much larger in B. hearnii sp.nov. than in B. compacta where it is hardly present at all ( Fig 4 View FIGURE 4 A, B); (b) most B. compacta males have small sensory spines lining much of the medial surface of the proximal antennomere, whereas this surface is smooth in B. hearnii sp.nov.; (c) in female, B. compacta lateral tumidities on its genital segments are significantly smaller than the large ones in B. hearnii sp.nov. ( Fig. 4 View FIGURE 4 C, D, 7F, G); (d) The anterior setae on endites 4–6 are different in the two species, in B. compacta there are three longer setae each with pectens of spines and two very short naked setae, whereas in B. hearnii sp.nov. there are two longer naked setae and three shorter setae with pectens ( Fig. 5 View FIGURE 5 A, B); (e) in B. compacta the medial endopodal setae are feathered and have 10–14 small spines basally whereas in B. hearnii sp.nov. the medial endopodial setae are short, naked and basally spineless ( Fig. 5 View FIGURE 5 A, B); and (f) B. compacta occurs in southeastern Australia, mainly in western Victoria ( Geddes, 1981) but including some lakes on the Monaro, NSW (see above), while B. hearnii n.sp, inhabits sites in southwestern Western Australia, both north and south of Perth.
Resting eggs of these two species are easily differentiated: those of B.hearnii sp.nov. have fewer depressions than eggs of B. compacta (<15 cf>25) and their walls are sharp compared to rounded in B. compacta ( Fig. 1 View FIGURE 1 D, F) (Timms and Lindsay, 2011).
Given the genital segment tumidities and projections in females and the ventral lamellar projection in the clasper apparatus of males (reminiscent of males of Parartemia — Timms, 2012), it most likely exhibits lock and key amplexus ( sensu Rogers, 2002). The male claspers would appress the genital segments perhaps either anterior or posterior to the dorsal pyramid shaped projections, to form a tight union. While all species of the Australian brine shrimp ( Parartemia ) use lock and key amplexus ( Rogers, 2002; Timms, 2011), B. hearnii sp.nov. is the only second known species in Branchinella to possibly use it, its close relative B. compacta being the other candidate. The advantage to it is not clear in the Unicup area as no other species of Branchinella is known from this area, but in the Coomberdale — Watheroo area, B. hearnii sp.nov. shares its known sites with B. erosa sp. nov.
Distribution and Ecology. B. hearnii sp.nov. occurs in a few hyposaline lakes to 8 g /L to the north of Lake Muir in southwestern Western Australia as well as in a few lakes, also hyposaline to 12 g /L, in the Coomberdale — Watheroo area north of Moora, Western Australia. In both areas the lakes in which it is found contain clear waters, turbidities <20 NTU. On the other hand its near relative, B. compacta , while also tending to occur in hyposaline lakes, lives in turbid waters often> 200 NTU.
| WAM |
Western Australian Museum |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |