Lagorchestes conspicillatus, Gould, 1841
|
publication ID |
https://doi.org/ 10.5281/zenodo.6723703 |
|
DOI |
https://doi.org/10.5281/zenodo.6722528 |
|
persistent identifier |
https://treatment.plazi.org/id/03950439-9642-FFA7-6ABC-F31CF90B3AD9 |
|
treatment provided by |
Tatiana |
|
scientific name |
Lagorchestes conspicillatus |
| status |
|
45. View Plate 41: Macropodidae
Spectacled Hare Wallaby
Lagorchestes conspicillatus View in CoL
French: Wallaby a lunettes / German: Brillen-Hasenkanguru / Spanish: Ualabi liebre de anteojos
Other common names: Grass Pademelon, Spectacled Hare-wallaby; Barrow Island Spectacled Hare Wallaby (conspicillatus)
Taxonomy. Lagorchestes conspicillatus Gould, 1842 View in CoL ,
“Barrow Island, on the north west coast of Australia.”
Variation in size and coloration across the species’ range warrants a detailed morphological and genetic study. Two subspecies recognized.
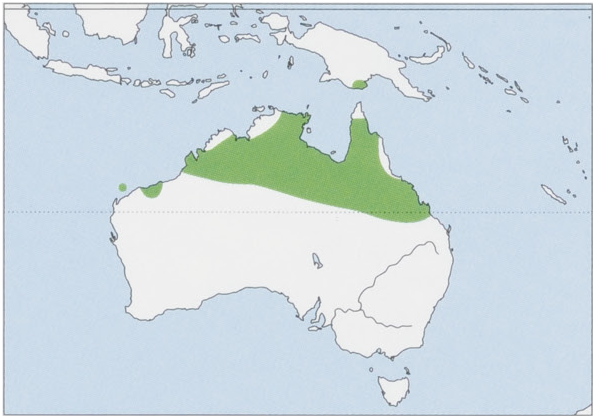
Subspecies and Distribution.
L.c.conspicillatusGould,1842—BarrowI,WesternAustralia.
L. c. leichardti Gould, 1853 — N mainland Australia and SW Papua New Guinea. View Figure
Descriptive notes. Head-body 41-49 cm (males) and 39.2-50 cm (females), tail 46-2— 53 cm (males) and 42.4-51.2 cm (females); weight 3-3—4-8 kg (males) and 2.1-4.3 kg (females). A large, stocky, shaggy hare wallaby with distinct orange to rich rufous rings around eyes. Dorsally light to dark brown, heavily grizzled with white and rufous; pale gray to white ventrally. Upper lip white. Ears long and pointed, back of ears sometimes rufous. Pale gray hip stripe and limbs. Tail pale gray, sometimes brownish, sparsely furred, and darkening distally in some individuals. Coastal Queensland populations are overall paler and less rufous. Barrow Island population ( L. ¢. conspicillatus ) is smaller, darker brown and less brightly colored. Diploid chromosome number is 15 in males and 16 in females.
Habitat. Open forest and woodland with grassy understory, tall shrubland with tussock grass understory, as well as tussock and hummock grasslands. Shelter sites of dense shrubs, grass tussock, or hummocks within 50 m of more open feeding areas are preferred on mainland; dunes and floodout flats preferred on Barrow Island.
Food and Feeding. Selectively feeds on leaves of 24 species of grasses, forbs, and shrubs. Constantly consumed grasses regardless of availability, and consumed forbs more opportunistically as availability changed at mainland sites. Seeds, fruit, browse, and succulents also significant dietary components in some locations or at certain times of year. On Barrow Island, consumes mostly forbs and small shrubs, as well as spinifex (7 Triodia , Poaceae ) leaf tips and seed-heads. Does not need access to free water.
Breeding. Females reach sexual maturity at about twelve months and males at 16 months. Females breed throughout year, producing one young per pregnancy and up to two young per year. On Barrow Island there are peaks of births in autumn and spring. Females exhibit embryonic diapause and post-partum estrus, usually mating shortly after giving birth. The estrous cycle is 30 days and gestation 29-31 (mean 30) days. Young spend around five months in the pouch and are weaned by seven months.
Activity patterns. Nocturnal, spending the day in a shallow to deep scrape dug under a large grass tussock or spinifex hummock, emerging at night to feed. Observations in central Queensland revealed that both sexes were more active in the first half of night.
Movements, Home range and Social organization. In central Queensland, home ranges (100% minimum convex polygon) averaged 260 ha for males and 110 ha for females, with relatively exclusive core areas (50% harmonic mean) of 40 ha for males and 23 ha for females. Home ranges may, however, be considerably smaller on Barrow Island. Daytime resting sites were located mostly within core areas. Individuals maintain multiple daytime shelters in their home range, and same shelter site is rarely used on consecutive days. Solitary, although feeding aggregations of up to three individuals may occasionally be seen.
Status and Conservation. Classified as Least Concern on The IUCN Red List. Barrow Island population is listed as vulnerable in Australia. Although the Spectacled Hare Wallaby remains widespread and locally common in some areas, it has declined significantly through much of its range. Populations assumed to be of the nominate conspicillatus from Hermite Island and Trimouille Island, Montebello Group, Western Australia, are now extinct. The Spectacled Hare Wallaby is now rare in the Pilbara and Kimberley regions of Western Australia, and appears to be extinct in Great Sandy Desert and southern Northern Territory. The cause of decline in arid areas appears to be predation by introduced Red Fox (Vulpes vulpes), increased fire frequency preventing formation of the large spinifex hummocks required for diurnal shelter, and habitat degradation by introduced herbivores. In eastern and central Queensland,significant areas of habitat have been lost to clearing for agriculture. Little high-quality habitat occurs in protected areas, and most of this species’ range is subject to cattle grazing. Spectacled Hare Wallabies are able to persist on cattle stations with conservative stocking rates and appropriate fire regimes. Many populations in Queensland and Northern Territory, however, continue to be threatened by habitat loss (clearing) and degradation (burning, logging, cattle grazing), which can also increase the vulnerability of individuals to predation by Dingoes (Canis lupus dingo). The Trimouille and Hermite island populations in Western Australia became extinct by 1912 and 1950, respectively, as a result of feral cat (Felis catus) predation. In 2010, Spectacled Hare Wallabies were reintroduced to Hermite Island by means of translocation of individuals from nearby Barrow Island. Additional research on taxonomy, distribution, abundance, general ecology, dispersal, and impact of threats is required. Very little is known ofthis species’ status in New Guinea.
Bibliography. Bakker & Bradshaw (1989), Burbidge & Johnson (2008), Burbidge et al. (1988), Courtenay (1993), Gould (1853), Groves (2005b), Hayman (1989), Hitchcock (1997), Horsup & McCosker (1992), Ingleby (1991a), Ingleby & Westoby (1992), Johnson, B. (2010), Johnson, PM. (1993, 2003), McCosker (1997), Menkhorst & Knight (2001), Menzies (2011), Short & Turner (1991), Winter, Woinarski & Burbidge (2008), Woinarski et al. (2014t, 2014u, 2014v).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Lagorchestes conspicillatus
| Russell A. Mittermeier & Don E. Wilson 2015 |
Lagorchestes conspicillatus
| Gould 1842 |