Thylogale stigmatica (Gould, 1860)
|
publication ID |
https://doi.org/10.5281/zenodo.6723703 |
|
DOI |
https://doi.org/10.5281/zenodo.6722386 |
|
persistent identifier |
https://treatment.plazi.org/id/03950439-9655-FFB1-6F60-F6B7FE553D93 |
|
treatment provided by |
Tatiana |
|
scientific name |
Thylogale stigmatica |
| status |
|
12. View Plate 37: Macropodidae
Red-legged Pademelon
Thylogale stigmatica View in CoL
French: Thylogale a pattes rousses / German: Rotbeinfilander / Spanish: Pademelon de patas rojas
Other common names: Pademelon, Scrub Wallaby
Taxonomy. Halmaturus stigmaticus Gould, 1860 ,
“ Point Cooper, on the north-east coast of Australia ,” Queensland, Australia.
Recent genetic studies have revealed a complex pattern of differentiation across the species’ range, including evidence of hybridization between some lineages. Populations of south-eastern Australia (wilcoxt) are genetically the most distinct and may represent a separate species. In contrast, populations from Cape York Peninsula (coxenii) and southern New Guinea (oriomo) are only weakly differentiated and may not warrant recognition as subspecies. A comprehensive study of morphological variation within this species and additional genetic sampling are needed in order to resolve these matters. Four subspecies currently recognized.
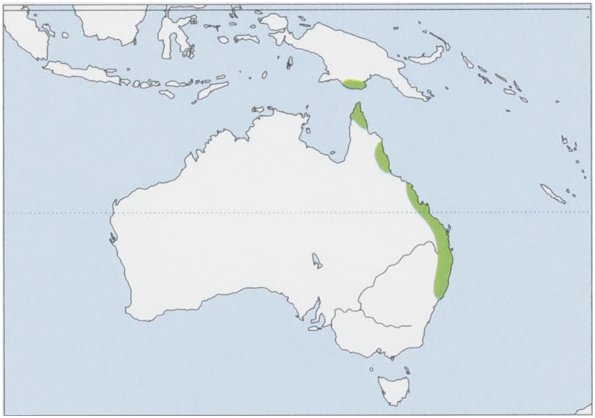
Subspecies and Distribution.
T. s. stigmatica Gould, 1860 — NE & C coastal Queensland from Cooktown to Proserpine, NE Australia.
T. s. coxenii Gray, 1866 — Cape York Peninsula N of Coen, NE Queensland, Australia.
T. s. oriomo Tate & Archbold, 1935 — Trans-Fly region of S Papua New Guinea.
T. s. wilcoxi McCoy, 1866 — C & SE coastal Queensland S to Wyong, on C coast of New South Wales, Australia. View Figure
Descriptive notes. Head-body 47-536 cm (males) and 38.6-52 cm (females), tail 37.2-47.3 cm (males) and 30.1-45.5 cm (females); weight 3.7-6.8 kg (males) and 2.5-4.2 kg (females). Medium-sized, short-tailed, brightly colored pademelon. T. s. stigmatica is dark gray-brown dorsally, grading to dark gray on shoulders and upper arms, and to rich rufous on flanks and legs; off-white ventrally. Face and arms also often rufous. Pale cheek stripe and faint but broad, pale yellow hip stripe. A broad indistinct dark mid-dorsal stripe runs from head to middle of back. Limbs largely hairless on inside. Tail gray-brown, sparsely furred laterally and paler ventrally; white tail tip occasionally present. 7. s. wilcoxi is similar, but differs in having longer fur, being less brightly colored, and pale gray ventrally, this sometimes extending to inside of limbs; hip stripe and mid-dorsal stripe are absent or much reduced, face is often rufous, but arms are usually light gray; neck typically gray, but is rufous in some specimens. 7. s. coxenii has shorter fur, is more grizzled, sandy brown dorsally, white ventrally, with sandy rather than rufous legs, but a more prominent white hip stripe and darker tail. 7_ s. oriomo is similar to 1. s. coxenii, but with hip stripe and ventral fur pinkish. Diploid chromosome number of T. s. stigmatica and 1. s. wilcoxii is 22, although the two differ in X chromosome morphology.
Habitat. Tropical and subtropical rainforest and adjacent wet sclerophyll forest, gallery forest, dry rainforest, and vine thickets.
Food and Feeding. Feeds on leaves,fruit, and seeds from a wide variety of forest plants (trees, shrubs, vines, ferns), and sometimes fungi (both hypogeous and epigeous). Leaves and fruit are taken directly or picked up from forest floor. In north of range, regularly feeds on grasses at forest edge.
Breeding. Females reach sexual maturity at about eleven months and males at c.15 months. Females are continuous breeders, producing one young per pregnancy, and births occur throughout year. Females exhibit embryonic diapause and post-partum estrus, usually mating within twelve hours of giving birth. Estrous cycle is 29-32 (mean 31) days and gestation 28-30 (mean 29-5) days. Young spend around six months in the pouch and are weaned at about eight months. After permanent pouch emergence, young accompanies mother as a young-at-foot until after weaning. Adult males are significantly larger than adult females, suggesting intense competition among males for access to females.
Activity patterns. A shy and cryptic species, rarely moving far from dense cover. Active both day and night within forest, although often rests in early afternoon and around midnight. In south of range, rarely seen outside forest. In north, frequently moves to forest edge after dark to feed on grasses in more open areas until just before dawn.
Movements, Home range and Social organization. Rarely ventures more than 70 m from forest edge and rapidly returns to forestif disturbed, often utilizing well-established runways. In north-east Queensland in fragmented forest, home ranges (95% minimum convex polygon) are small (4 ha) and of similar size for males and females. Individuals have spatially distinct larger diurnal ranges within forest and smaller nocturnal ranges on forest edge, moving rapidly between the two ranges just after dusk and prior to dawn. Solitary, although small feeding aggregations occur underfruiting forest trees and on pasture adjacent to forest.
Status and Conservation. Classified as Least Concern on The IUCN Red List. Although this species has lost considerable areas of habitat in eastern Australia owing to clearing for agriculture, it remains widely distributed and relatively common at least in northern parts of range. It occurs in protected areas in Australia but not in New Guinea. Populations in New Guinea are potentially threatened by overhunting by local people. Although, in north-east Queensland, the Red-legged Pademelon has benefited from some forest fragmentation and the establishment of pasture adjacent to forest,it is vulnerable to further habitat loss, predation by domestic/feral dogs, and roadkill. It is much less common in south of range and these populationsare likely to be threatened by predation by dogs and introduced Red Fox (Vulpes vulpes). Additional research on taxonomy, abundance, general ecology, and impact of potential threats is required, especially in south of range.
Bibliography. Burnett & Ellis (2008), Eldridge et al. (2011), Gould (1860), Gray (1866), Hayman (1989), Jarman & Phillips (1989), Johnson (2003), Johnson & Vernes (1994, 2008), Macqueen et al. (2010), McCoy (1866), Menkhorst & Knight (2001), Tate & Archbold (1935), Troughton (1967), Vernes (1995), Vernes & Trappe (2007), Vernes etal. (1995).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
SubClass |
Metatheria |
|
Order |
|
|
SubOrder |
Macropodiformes |
|
Family |
|
|
Genus |
Thylogale stigmatica
| Russell A. Mittermeier & Don E. Wilson 2015 |
Halmaturus stigmaticus
| Gould 1860 |