SCHIZOPORELLOIDEA Jullien, 1883
|
publication ID |
https://doi.org/10.1080/00222930802109140 |
|
publication LSID |
lsid:zoobank.org:pub:2B6D5D3B-8F6D-4F0C-A377-784C4CBCF7E2 |
|
persistent identifier |
https://treatment.plazi.org/id/0395A723-FFAE-FFFE-FEA5-FBB5FE89ECB9 |
|
treatment provided by |
Felipe |
|
scientific name |
SCHIZOPORELLOIDEA Jullien, 1883 |
| status |
|
Superfamily SCHIZOPORELLOIDEA Jullien, 1883 View in CoL
Family ESCHARINIDAE Tilbrook, 2006 View in CoL
Genus Herentia Gray, 1848 View in CoL
Herentia Gray 1848, p. 148 View in CoL .
Mastigophora Hincks 1877, p. 527 View in CoL .
Mastigophorella Bassler 1953, p. 220 .
Herentia: Buge 1957, p. 234 View in CoL ; David and Pouyet 1978, p. 168.
Type species. Lepralia hyndmanni Johnston, 1847 .
Revised diagnosis
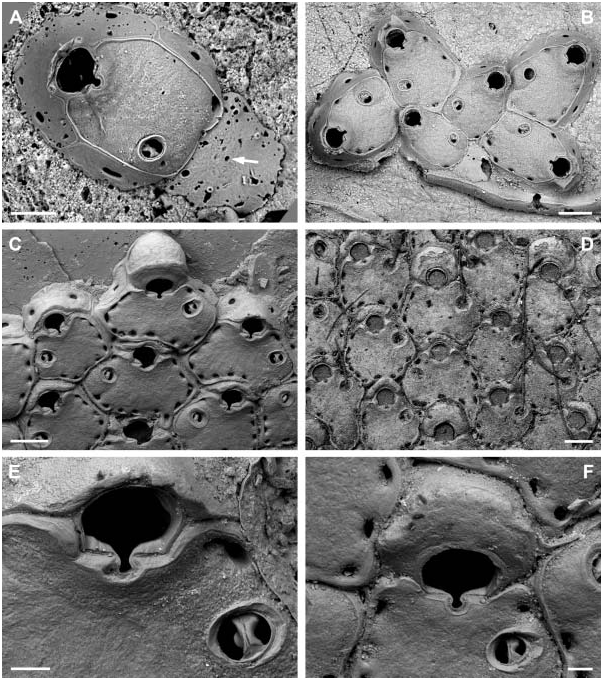
Colony encrusting unilaminar. Frontal wall cryptocystidean, occasionally with a smooth gymnocystal area surrounding the orifice; frontal shield more or less flat, heavily calcified and imperforate except for a row of large marginal pores. Lateral walls extensive, divided into distinct lateral and distal areas containing relatively few septular pores, basal pore chambers present. Basal wall only marginally calcified.
Orifice dimorphic, with condyles, a sinus and an immersed distal shelf, distolateral orifice border in autozooecia with or without a thickened rim of calcification originating from the distal basal pore chambers. Oral spines present or absent.
Ooecium independent of, but partly immersed by, distal zooid, imperforate, exposed surface a flattened and thickened rim developing during ontogeny, proximal ooecium margin affixed to proximolateral orifice corners, aperture at an acute angle to colony surface, closure of ovicell subcleithral, distal rim of primary orifice retained in ovicellate zooecia.
Avicularium interzooidal, single, proximolateral to orifice on right or left on a large flat cystid, replacing a basal pore chamber and being connected with associated zooid via several communication pores; rostrum a round or oval, variably broad and sloping rim with a smooth surface; mandible setiform; crossbar complete, thick, usually bilateral symmetrical, variably sculptured.
Early astogeny: first autozooid budded distally, from which a single proximolateral zooid is formed at a 90 ° angle before distolateral, lateral and proximal periancestrular zooids are produced, periancestrular area therefore asymmetrical, colony growth usually proceeds faster in distal than in proximal directions whereas overgrowth of the ancestrula by periancestrular zooids occurs during relatively early astogeny.
Remarks
We here follow Buge (1957) and David and Pouyet (1978), as well as Tilbrook (2006, p. 251) and D. P. Gordon (pers. comm. 2007), who considered Herentia to be distinct from Escharina Milne Edwards, 1836 . Besides differences in the general zooecium morphology, such as the flat and comparatively smooth frontal wall, Herentia is characterized by extensive and thick vertical walls containing few septular pores. In contrast, the frontal wall is generally convex and granular, and the vertical walls are reduced and contain a distinctly greater number of septular pores in Escharina . From the septular pores an additional, characteristic, distal wall is added to autozooecia in Herentia that may be expressed at the colony surface as a thick rim framing the distolateral orifice margin in some species (the thickened rim was, however, only observed in taxa in which oral spines are absent in adult zooecia). The exact origin and formation of this distal rim needs to be examined by sectioning live material and by studying the zooidal ontogeny, which was not possible during this study of dried specimens. Further differences are found in the ancestrula as well as the early astogenetic budding pattern, in the formation of the ovicell, and in avicularium morphology.
The ancestrula of the type species Herentia hyndmanni ( Johnston, 1847) , apparently a kenozooid, is here reported for the first time ( Figure 1A, B View Figure 1 ; see description of H. hyndmanni below). However, because only five colonies of Mediterranean deep-water representatives have yielded this curious kind of ancestrula, we chose not to include this feature in the generic definition of Herentia . It is seldom observed due to overgrowth by periancestrular zooids and, if exposed, often completely or partially damaged owing to its weak calcification. The ancestrula is almost entirely composed of an untextured gymnocyst apart from a wide circle of spines and a tiny central hole, most likely the remnant of an opesium (see below). A cryptocyst is obviously absent. It differs considerably from the other known ancestrulae in species of Escharina , as the type species E. vulgaris ( Moll, 1803) has a tatiform ancestrula, in which an extensive opesium is bounded by a narrow proximolateral cryptocyst and is surrounded by about nine spines (Hayward and Ryland 1999, p. 236; Hayward and McKinney 2002, p. 72). The ancestrula of E. johnstoni ( Quelch, 1884) , in contrast, has an extensive gymnocyst leading up to a central circular rim without spines, while the extensive cryptocyst is surrounding a small round opesium (Hayward and Ryland 1999, p. 234) (see also the Discussion below). The early astogenetic, asymmetrical budding pattern is peculiar, with a single lateral zooid forming at a 90 ° angle from the first autozooid, from which then two zooids are budded, again at a 90 ° angle ( Figure 1B View Figure 1 ). The distolateral zooid is formed together with the first autozooid and both then give rise to another distolateral zooid at a 90 ° angle. Only then is the zooid on the other lateral side of the first zooid formed (not yet present in the figured colony), with a growth polarity in the opposite direction, i.e. proximally. This budding pattern was observed in most Herentia species , and presumably also occurs in Therenia (see below), but differs from those of other Escharinidae in which two distolateral zooids are usually budded from the ancestrula (see Discussion).
There is no information available concerning ovicell formation in Herentia . The ooecium seems to be a homologous structure of the thickened rim framing the orifice in autozooids, and thus formed by the maternal zooid, but thin sectioning of material with the soft tissue preserved is needed before a conclusion on its origin can be drawn. Nevertheless, the ovicell forms independent of, but is partly immersed by, the distal zooid in Herentia , whereas it usually rests on the distal zooid’s frontal wall in Escharina (but see Discussion). Closure of the ovicell is subcleithral (see Ostrovsky, in press). The aperture in ovicellate zooecia is formed by the proximal rim of the primary orifice and the proximal ooecium margin, on which the operculum rests during brooding. When the ovicell is empty the operculum may be lowered onto the retained distal margin of the primary orifice inside the ooecium.
Another feature distinguishing Herentia from Escharina is the presence of a single, orbicular, frontal avicularium, which is situated on a large cystid that is more or less level with, and completely incorporated into, the frontal wall. The rostrum is developed as a broad oval band slightly sloping towards the centre, while the crossbar is variably sculptured and extremely thick. In contrast, avicularia in Escharina are usually paired, distinctly smaller, and project above the surface to some extent. Furthermore, the avicularium is rather of the ‘‘normal’’ type, i.e. the rostrum is more or less triangular (albeit distally truncate in certain species to accommodate the long setiform mandible) and the crossbar is rather thin and simple. The difference between these avicularium morphotypes is revealed in a twodimensional movement of the setiform mandible in Escharina , whereas it is threedimensional in Herentia .
There are only two established recent species belonging to Herentia : the type species H. hyndmanni , which has been reported from Great Britain to Madeira and the Mediterranean Sea, as well as H. thalassae David and Pouyet, 1978 from the north-western Spanish shelf, which has never been cited again after its discovery.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
SCHIZOPORELLOIDEA Jullien, 1883
| Berning, Björn, Tilbrook, Kevin J. & Rosso, Antonietta 2008 |
Herentia : Buge 1957 , p. 234
| Buge E 1957: 234 |
Mastigophorella
| Bassler RS 1953: 220 |
Mastigophora
| Hincks T 1877: 527 |
Herentia
| Gray JE 1848: 148 |