Cettia haddeni, Lecroy & Barker, 2006
|
publication ID |
https://doi.org/10.1206/0003-0082(2006)3511[1:ANSOBF]2.0.CO;2 |
|
persistent identifier |
https://treatment.plazi.org/id/03967B65-FF8D-8A4D-8BB2-FE10FD39FD71 |
|
treatment provided by |
Carolina |
|
scientific name |
Cettia haddeni |
| status |
sp. nov. |
Cettia haddeni , new species
English name: Odedi
DIAGNOSIS: Adults ( one male, one unknown sex) with body mass greater than 21 grams —about four grams greater than any other Cettia known (table 1; Dunning 1993). All three individuals, including an immature of unknown sex, have bill width at the posterior margin of the nostril $ 7 millimeters, at least 2 millimeters greater than any other Cettia measured (table 1). Underparts darker than any other Cettia (closest being C. ruficapilla funebris , see below).
HOLOTYPE: AMNH 835234 About AMNH , adult male, collected between Kupei and Moreni villages, 1000 m, Crown Prince Range , Bougainville Island , North Solomons Province , Papua New Guinea, on 16 September 2000, prepared by Andrew Mack (no. 1380). Flattened wing 66.0 mm, tail [43.0] worn, tarsus 29.0, bill length from base 19.0, bill length from anterior edge of nostril 10.0, bill width at posterior edge of nostril 7.0. Colors of soft parts: tarsus yellowish-ochre (straw), browner on front; base of mandible yellowish-ochre, rest of bill dark brown; inside of mouth yellow. Testes 7 × 4 mm; skull ossified; no molt; tissues preserved in EtOH. The weight at time of skinning was recorded as 25 g, but the specimen had been frozen for some time.
PARATYPES: AMNH 833347 About AMNH , adult sex?, Crown Prince Range , Bougainville Island , North Solomons Province , Papua New Guinea, January 2000, prepared by Andrew Mack (no. 1218). Flattened wing 66.0, tail [43.0] worn, tarsus 29.0, bill length from base 19.0, bill length from anterior edge of nostril 10.0, width of bill at posterior edge of nostril 8.0. Colors of soft parts: tarsus brown with yellowish cast to digits; bill dark brown, base of lower mandible paler, yellow-brown cast to tomentia. Skull 85% ossified; tissue preserved in EtOH. The weight at time of skinning was recorded as 24.5 g, but the specimen had been frozen for some time .
AMNH 836189 About AMNH , immature sex?, Crown Prince Range , 1400 m, Bougainville Island , North Solomons Province , Papua New Guinea, 11 August 2001, prepared by Andrew Mack , no. 1571. Flattened wing [60.0] molt, tail missing, tarsus 26.5, bill length from base 19.0, bill length from anterior edge of nostril 10.0, width of bill at posterior edge of nostril 7.0. Colors of soft parts: iris dark, bill very dark brown, almost black, mouth lining dull yellow brown. Skull 10% ossified; tissue preserved in EtOH. This specimen had also been frozen for some time .
DESCRIPTION OF THE HOLOTYPE
Upperparts: Head dark chestnut with faint lighter streaks in the center of the feathers, back very dark brown with wings and tail more chestnut, ten rectrices, wings very rounded and unmarked (primary 10 half the length of 9, primary 9 ten mm shorter than 8, primary 8 five mm shorter than 7, primary 7 slightly shorter than 6, primaries 6 and 5 subequal, remainder of the primaries differ little in length from 6 and 5, those being slightly longer). Head: Sides of head brown with lighter streaks in the center of the feathers, dark loral spot, no eye-stripe, but feathers over the eye are slightly lighter; rictal bristles prominent; bill strong, broadened at base. Underparts with black feather bases tipped with gray, giving a mottled appearance, throat lighter. Flank feathers long and brownish olive; both tarsi and phalanges noticeably elongate. Paratype AMNH 833347 is similar, but feathers missing on the underparts make this specimen appear even blacker below. The almost identical measurements of these two specimens lead us to believe that it is perhaps also a male, as females are usually considerably smaller in this genus (see table 1). Paratype AMNH 836189 has very similar plumage. The presence of wing and body molt and the largely unossified skull indicate that this specimen is immature. The shorter tarsus may also indicate that this is a female and that there is a sexual size difference in this species as in others in the genus, as tarsus length in immature birds usually equals that of the
TABLE 1 Measurements of Cettia haddeni , C. parens , C. ruficapilla , and C. carolinae (from Rozendaal, 1987: 187) Measurements in brackets indicate wear on the measured specimen, means for samples greater than two and sample sizes given in parentheses. Total culmen 5 culmen measured from base at skull; Culmen 5 culmen measured from anterior margin of nostril; Posterior bill width 5 width measured at posterior margin of the nostril.
adult in ground dwelling species (M.L., personal obs.). The digits are also noticeably shorter in this specimen.
RELATED SPECIES
When Ramsay (1876) named Vitia and its type species, V. ruficapilla , he noted that it had only 10 rectrices. Mayr (1935: 4–5, 1936: 15– 16) did not mention the number of rectrices when he named V. parens , but it also has 10 (personal obs.). Delacour (1942) noted that species in the genus Cettia have 10 rectrices. Later, Orenstein and Pratt (1983) discussed the relationships of the southwest Pacific genera Vitia and Psamathia and concluded, based on song structure, egg color, and external morphology, that these genera with 10 rectrices should be included in Cettia . This arrangement has been followed by subsequent authors, including Watson et al. (1986: 11), Sibley and Monroe (1990: 609) and Dickinson (2003: 579). This new species from Bougainville also possesses the 10 rectrices of Cettia (sensu lato).
Geographically, the closest representative of Cettia is C. parens from Makira Island, Solomon Islands. Compared with that species, the Bougainville bird is larger with a shorter tail. Its upperparts are a darker, richer brown (not olive-brown). Below it lacks the yellowish brown tint of parens , the feather tips being gray. Because the gray tips are not as broad, the black (not gray) feather bases are much more apparent. The flanks are a much darker brownish olive. The rictal bristles are more prominent, and the tarsi are longer and heavier with longer digits. Mayr (1936: 15– 16) noted that the juvenile of ‘‘ Vitia ’’ parens (younger than the immature specimen of haddeni ) was very different from the adult, with the ‘‘middle of throat yellowish; breast, belly, and flanks grayish olivaceous, lower belly and under tail-coverts with a brownish wash; forehead and crown fuscous; back, wings, and tail fuscous brown; under wingcoverts yellowish; the whole plumage very soft.’’ Interestingly, the upperparts of this bird are much more similar to all three specimens of C. haddeni than to adult parens , but the crown and sides of the face are almost entirely fuscous, showing only a very few narrow, lighter tips on the forehead feathers. It is very different from both forms in the coloration of the underparts. Orenstein and Pratt (1983: 190) called attention to similarities in the coloration of the underparts of this juvenile specimen to the underparts of adults of C. annae .
Comparison with the four subspecies of Cettia ruficapilla from Fiji, shows that C. haddeni is most similar to C.r.funebris from Taveuni Island in color of the upperparts, but somewhat more brownish on the lower back and tail. The lighter shaft streaks present on the head of C. haddeni are lacking in funebris. The side of the face is browner than in funebris and there are only slightly lighter feathers over the eye, whereas funebris has a readily visible tan eyestripe. The underparts of haddeni appear much darker because the feather tips are darker and narrower and expose more of the blacker, rather than gray, feather bases. The flank feathers are longer and darker than those of funebris. Overall, haddeni appears much darker, almost melanistic, when compared with funebris, and it differs most strikingly in its shorter tail, its longer and heavier tarsus and longer phalanges, and its wider bill.
Cettia carolinae View in CoL from Yamdena Island in the Tanimbar Islands, South Moluccas, Indonesia ( Rozendaal, 1987) was found by Rozendaal to be morphologically closest to Cettia ruficapilla View in CoL , differing in details of coloration, and in possessing a marked sexual dimorphism and longer bill. Although specimens of C. carolinae View in CoL were not available for direct comparison, measurements given for C. carolinae ( Rozendaal, 1987: 187) View in CoL show that the wing, tail, and bill measurements of the holotype of C. haddeni approach those of carolinae View in CoL (table 1). However, tarsal measurements and body mass are much greater in haddeni .
MORPHOMETRIC ANALYSIS
We made standard external morphological measurements of C. haddeni as well as of the geographically and morphologically closest representatives of genus Cettia (table 1). The lengths of wing (flattened), tail, tarsus, and culmen (both from base of bill at the skull and from the anterior edge of the nostril) were measured to the nearest 0.5 mm. Individuals of both sexes of C. annae , C. parens , and four subspecies of C. ruficapilla ( funebris, castaneoptera, ruficapilla , and badiceps) were included (see table 1, fig. 2 View Fig , and appendix 1). In addition, we obtained comparable measurements (excepting bill width) of the recently described species C. carolinae from Rozendaal (1987). We made two quantitative comparisons of C. haddeni with other species of Cettia . Due to the lack of a positively identified female C. haddeni , and the poorer available sample of females from other species, these comparisons were limited to male measurements alone, treating both the holotype and the paratype AMNH 833347 of haddeni as males (their measurements are nearly identical, in any case).
In the first comparison, we performed a principal components analysis on the species means for the external measurements, as reported in Orenstein and Pratt (1983), together with those for the subsequently described C. haddeni and C. carolinae . In addition to C. ruficapilla , C. annae , and C. parens , the data presented by Orenstein and Pratt (1983) included the mainland forms C. fortipes , C. major , and C. diphone , as well as the Luzon endemic C. seebohmi (sometimes considered a subspecies of diphone ). The measurements reported in Orenstein and Pratt (1983) were taken differently from ours (e.g., they measured wing chord and exposed culmen) and included measurements of bill width and depth at the anterior edge of the nostril. In order to use their data, we obtained measures of wing chord, as well as of bill width and depth at the anterior edge of the nostril, from C. haddeni and C. carolinae (the latter courtesy of R. Dekker and H. van Grouw, National Museum of Natural History, Leiden; table 2). We excluded the measurement Orenstein and Pratt called ‘‘culmen total length’’ ( 5 exposed culmen). All variables were scaled to mean zero and unit variance, and the principal components calculated using the prcomp() function of R v1.9.0 ( R Development Core Team, 2004).
The analysis of species means (table 2) yielded two principal components with eigenvalues greater than one, which together explained 82% of the variation in the original set of six variables. The eigenvalues and eigenvectors are reported in table 3. Coefficients of the first component were of
TABLE 2
Mean Measurements of C. haddeni and C. carolinae for Comparison with Data of Orenstein and Pratt (1983) Sample sizes in parentheses. Both the type and AMNH 833347 were treated as males for this analysis. Data for C. carolinae provided courtesy of R. Dekker and H. van Grouw (National Museum of Natural History, Leiden). Wing chord 5 length of wing (unflattened); Anterior bill width 5 width measured at anterior margin of the nostril; Anterior bill depth 5 depth measured at anterior margin of the nostril.
identical sign and similar magnitude, suggesting its interpretation as a ‘‘general size’’ variable, while the second component primarily contrasted bill and tail length. Plotting of species values on these two components ( fig. 3 View Fig ) indicated that the two individuals of C. haddeni are among the most distinctive forms of Cettia investigated. In fact, C. haddeni had the third highest Euclidean distance from the origin of the component space (3.16), exceeded only by C. fortipes pallidus (3.85) and C. diphone canturians (3.71). On these components, C. carolinae (Tanimbar, Indonesia), C. parens ( Makira, Solomon Islands), and C. annae ( Palau) were closest to C. haddeni . Taking all components into account, C. haddeni was closest to C. ruficapilla funebris , followed by C. parens (not shown).
The second comparison made was a principal components analysis of individual external measurements from specimens reported in table 1 and appendix 1. For these analyses, culmen from base and culmen from anterior edge of nostril, the latter of which is a subset of the former, were made into two structurally independent variables by subtracting the latter from the former: the difference (the distance from the base of the bill to the anterior edge of the nostril) was defined as the ‘‘upper culmen’’, and was analyzed in lieu of the full culmen measurement. In this comparison, we used the bill width at the posterior margin of the nostril, rather than at the anterior, as this appeared to be measured more reliably on the specimens examined (widths for C. carolinae were provided courtesy of Dekker and van Grouw). As above, all variables were scaled to mean zero and unit variance, and the principal components calculated using prcomp(). The eigenvalues and eigenvectors are reported in table 4, and the individual values are plotted on selected components in figure 4 View Fig .
Analysis of the individual data (table 4) yielded a reduction of variables similar to the species mean analysis, with three components having eigenvalues greater than one, which together explained 82% of the original variance. The coefficients of the first component
TABLE 3 Results of Principal Components Analyses of Cettia Species Means All measurements except for C. haddeni and C. carolinae from Orenstein and Pratt (1983: 188–189).
suggested interpretation as a size variable (table 4), while the second component contrasted bill width and tail length, and the third component was primarily determined by the relative proportions of the bill proximal and distal to the anterior margin of the nostril. Plotting of individuals on the first two components ( fig. 4A View Fig ) indicated a clear separation of C. haddeni and C. annae from the remaining species. Component three appeared to correlate with intrapopulation variation within the C. ruficapilla complex, although
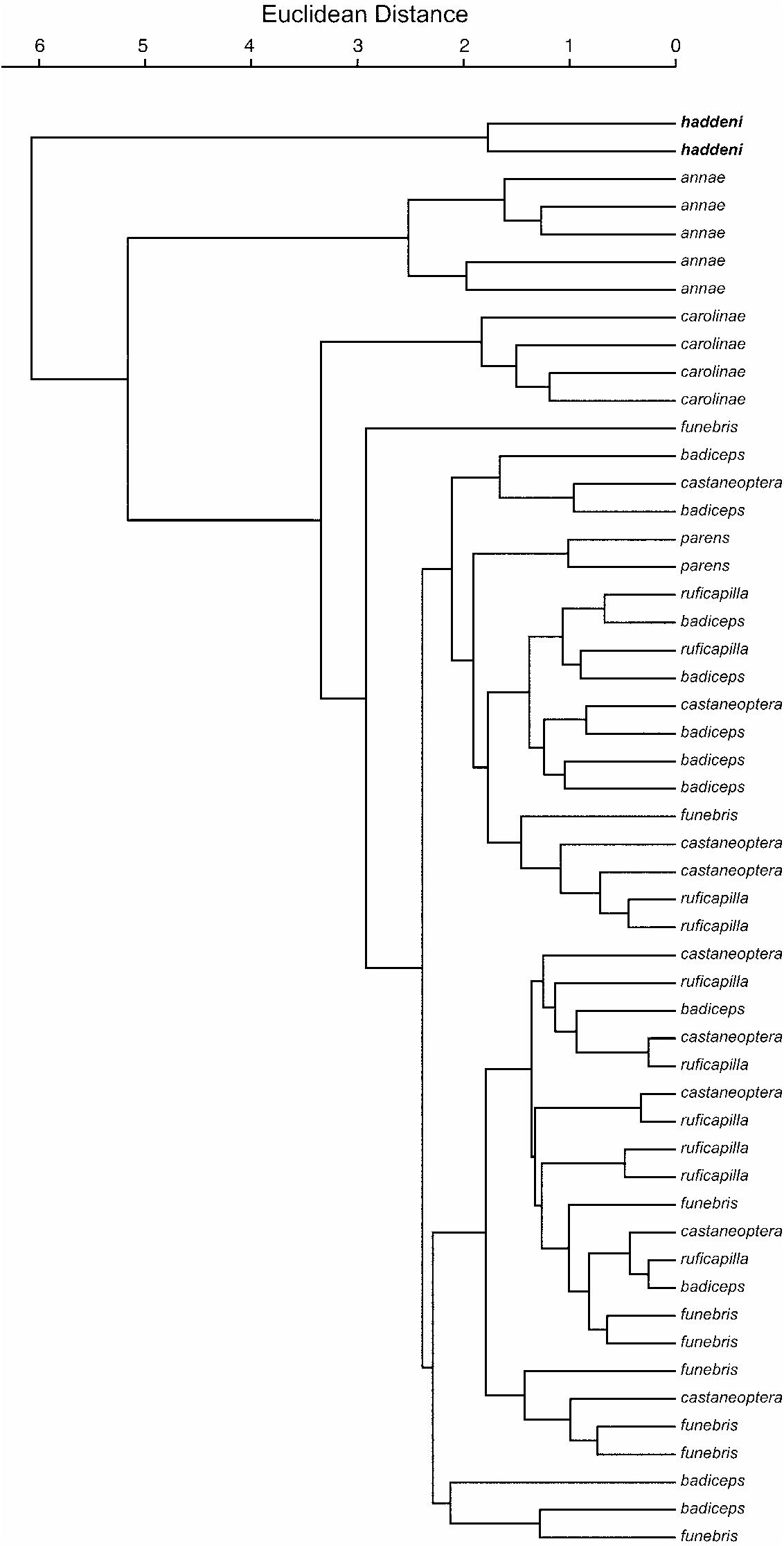
TABLE 4 Results of Principal Components Analyses of Individual Variation in External Measurements of Cettia Species clear distinctions among individual taxa were not apparent ( fig. 4B View Fig ). Although the fourth component explained relatively little additional variation (12.2%), it clearly distinguished C. carolinae from the other forms sampled ( fig. 4B View Fig ). Clustering analysis of individuals in component space yielded clear species clusters for C. annae , C. carolinae , and C. haddeni , but nested C. parens within variation among C. ruficapilla subspecies ( fig. 5 View Fig ). In this analysis, C. haddeni appeared to be the most distinctive of the forms sampled.
PHYLOGENETIC RELATIONSHIPS
Although the new species agrees with Cettia in its coloration and morphometry, we also sought to establish its relationships using molecular data. To this end, we obtained sequences from two genes from two of the three available samples. As a wide range of comparative material was available for the RAG1 locus (e.g., Barker et al., 2004; Beresford et al., 2005), we attempted to isolate this gene from all three samples. However, either handling of the samples in the field prior to freezing or the long period of storage at relatively high temperatures led to degradation of their DNA, and we were able to obtain only a partial sequence from the 3 9 end of the gene (GenBank accession DQ066452 View Materials ; 1182 bp, the region amplified by primers R21/ R24 and R23/R2I; Groth and Barrowclough, 1999) from a single sample (the holotype AMNH 835234; see Barker et al., 2002, for methods). Phylogenetic analysis of this partial sequence was performed (using PAUP* v4.0b10, equally weighted parsimony, TBR branch swapping after 50 random addition sequence replicates; Swofford, 2002), including previously published RAG1 sequences (as listed in Beresford et al., 2005). This analysis placed the partial RAG1 sequence as sister to Cettia brunnifrons ( fig. 6 View Fig ), and bootstrap analysis (100 replicates; Felsenstein, 1985) indicated strong support for this placement (found in 100% of replicates).
In addition to the nuclear sequence, we also obtained complete mitochondrial cytochrome b sequence from a second individual (the paratype AMNH 836189; see Barker, 2004 for methods, although the 5 9 primer used was L14857, 5 9 -AGGATCATTCGCCCTATCC- AT-3 9). We compared this sequence to Acrocephaline and Megalurine (sensu Sibley and Monroe, 1990) sequences available in GenBank (including samples from the genera Acrocephalus , Bradypterus , Cettia , Cisticola , Hippolais , Locustella , Megalurus , Orthotomus , Phylloscopus , Prinia , Seicercus , and Urosphena ). Three Asian species of Cettia ( cetti , fortipes , and diphone ) and Urosphena squameiceps were represented by sequences in the database. Of the sequences surveyed, the C. haddeni sequence clustered unambiguously (under both parsimony and distance criteria; results not shown) with seven haplotypes of Cettia diphone , at an average of 5.0% uncorrected sequence divergence. In order to estimate the relationship of C. haddeni to other island as well as mainland forms, we also obtained partial cytochrome b sequences from two individuals each of three additional species: C. ruficapilla , C. parens , and C. annae (see appendix 1 for individuals). For these individuals, genomic DNA was extracted from slivers of toe pad using the DNeasy extraction protocol (Qiagen, Maryland), modified by addition of 30 ML of 100 mg /mL dithiothreitol (ISC BioExpress, Utah) to the digestion mix, and by final DNA elution in 50 ML of elution buffer. Based on the complete C. haddeni sequence and other published Cettia sequences, taxonspecific primers were designed (table 5) to target 640 bp of cytochrome b. PCR conditions and sequencing protocols were the same as for tissues. All DNA extraction and PCR setup was performed in a lab and building separate from that used for other avian molecular work. The complete cytochrome b sequence of C. haddeni and the partial sequences for the remaining species have been deposited in GenBank (accession DQ066451 View Materials and accessions DQ288966 View Materials - DQ288971 View Materials ).
The novel Cettia sequences were analyzed in conjunction with the continental species represented in GenBank, and Urosphena , using two sequences of Aegithalos and Psaltriparus as outgroups ( fig. 6 View Fig , and unpublished data; see fig. 7 for GenBank accessions). Parsimony analysis of the 640 bases obtained from the skin samples yielded a single most-parsimonious tree (not shown; L 5 386, CI 5 0.65, RI 5
TABLE 5 Primers Used in Amplification of Cytochrome b from Museum Skin Specimens
0.81). Likelihood model fitting on this tree suggested analysis with the HKY+ C model ( Hasegawa et al., 1985) of evolution (estimat- ed using DT-ModSel; Minin et al., 2003). Figure 7 shows a phylogram of the maximum likelihood estimate of relationships among the sampled species (from a heuristic search with 10 random sequence additions and TBR branch swapping), with support for individual nodes based on parsimony and likelihood bootstrap (1000 and 200 replicates, respectively), and a Bayesian analysis of the data using the same model (default priors, two runs with four incrementally heated chains run for 2? 10 6 generations, discarding 5? 10 5 generations prior to the chains achieving stationarity). This topology differs from the parsimony tree in its arrangement of island forms ( C. annae and parens switch positions), and in recovering a monophyletic Cettia (parsimony places C. cetti with Urosphena ). Support for the relationship of C. haddeni with Cettia was strong (99% bootstrap under parsimony and 1.00 estimated Bayesian posterior). Within the genus, C. haddeni was recovered as part of a monophyletic group of island forms (including C. ruficapilla , parens , and annae ), which was sister to the continental C. diphone . Support for the C. diphone /island clade was strong with parsimony and Bayesian analysis (99% and 1.00), but much weaker under maximum likelihood (54%), and monophyly of the island clade was weakly supported (68% and 50% for parsimony and likelihood respectively, estimated posterior 0.73). Although support for monophyly of C. diphone was good (85 and 69% under parsimony and likelihood, estimated posterior 1.00), the two subspecies (the migratory mainland C.d. borealis, and sedentary Japanese C. d. cantans) were significantly differentiated (separated at 2.5% uncorrected sequence divergence) and monophyletic ( Nishiumi and Kim, 2004). Divergence among the island forms was substantial, averaging 4.1% among species, compared to 5.1% between the island forms and C. diphone , and 0.2% polymorphism within the island forms with multiple sequences available.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.