Macrobiotus halei, Bartels, Paul J., Pilato, Giovanni, Lisi, Oscar & Nelson, Diane R., 2009
|
publication ID |
https://doi.org/10.5281/zenodo.186100 |
|
DOI |
https://doi.org/10.5281/zenodo.5626064 |
|
persistent identifier |
https://treatment.plazi.org/id/03974A7F-FFED-FFF8-33AF-30306562BE8D |
|
treatment provided by |
Plazi |
|
scientific name |
Macrobiotus halei |
| status |
sp. nov. |
Macrobiotus halei sp. nov.
Figs. 4–7 View FIGURE 4 View FIGURE 5 View FIGURE 6. A View FIGURE 7. A
Type locality: Brushy Mountain ATBI Plot ( W 83o 25.9307’, N 35o 40.5596’, 1468 m asl), GSMNP, Sevier County, Tennessee, USA. In soil and leaf litter in a heath bald community (dominant species Rhododendron maximum and Kalmia latifolia ).
Material examined: Holotype: Brushy Mountain. Paratypes: Brushy Mountain: 2 adults and 3 eggs (2 with fully developed embryos); Cataloochee: 7 specimens and 1 egg (with fully-developed embryo).
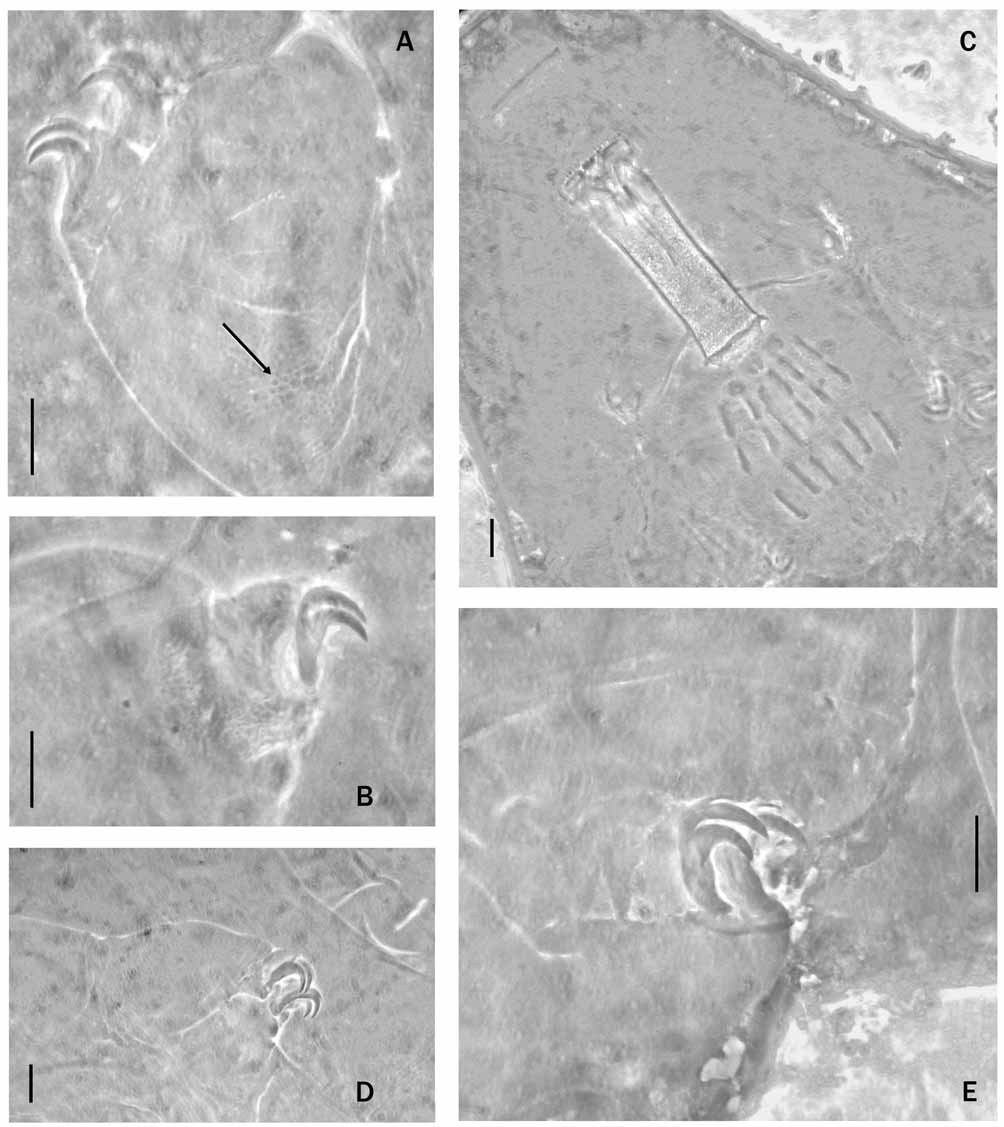
Species diagnosis: Colorless; cuticle with very small tubercles, quite difficult to see; very fine granules present on all legs, more evident on legs IV; eyes absent. Buccal armature in oral cavity of richtersi - type; buccal tube wide; stylet supports inserted on the buccal tube at 81.0–82.7% of its length; three macroplacoids and a microplacoid (far away from the third macroplacoid) present. Claws of the hufelandi - type; accessory points and lunules present. Eggs with truncated conical processes with a reticular design; egg shell areolated; areolae sculptured.
Description of the holotype: Body length 504.0 µm; colorless; eyes absent; cuticle with very small circular or elongated tubercles, more visible on the dorsal and lateral body wall, but very difficult to see ( Fig. 4 View FIGURE 4 A, B); smaller granules present on all legs ( Fig. 4 View FIGURE 4 B), but more evident on hind legs, especially in larger animals. Mouth terminal with 10 peribuccal lamellae; buccal armature in oral cavity well-developed ( Fig. 4 View FIGURE 4 C) with anterior band of small teeth, posterior band of triangular teeth (some of them fused), and three dorsal and three ventral transverse ridges. Some supplementary teeth present between the ring of triangular teeth and the transverse ridges on the ventral wall of the buccal cavity. Buccal tube 62.3 µm long and 17 µm wide ( pt = 27.3); stylet supports inserted on the buccal tube at 81.6% of its length ( pt = 81.6). Pharyngeal bulb with apophyses, three rod-shaped macroplacoids and a small microplacoid (with antero-lateral wings) far away from the third macroplacoid, a characteristic typical of species in the richtersi group. First macroplacoid 10.9 µm long ( pt = 17.5), the second 7.8 µm ( pt = 12.5), the third 12.1 µm ( pt = 19.4), the microplacoid 3.9 µm ( pt = 6.3); entire placoid row 47.9 µm ( pt = 76.9); macroplacoid row 37.1 µm long ( pt = 59.6).
Claws, of the hufelandi - type, well-developed ( Fig. 4 View FIGURE 4 D, E), with evident accessory points on the main branches. Internal and external claws on the third pair of legs 13.5 µm long ( pt = 21.7) and 14.7 µm long ( pt = 23.6), respectively; anterior and posterior claws on the hind legs 16.3 µm long ( pt = 26.2) and 17.4 µm long ( pt = 27.9), respectively. Other claws not measurable due to their unfavorable orientation.
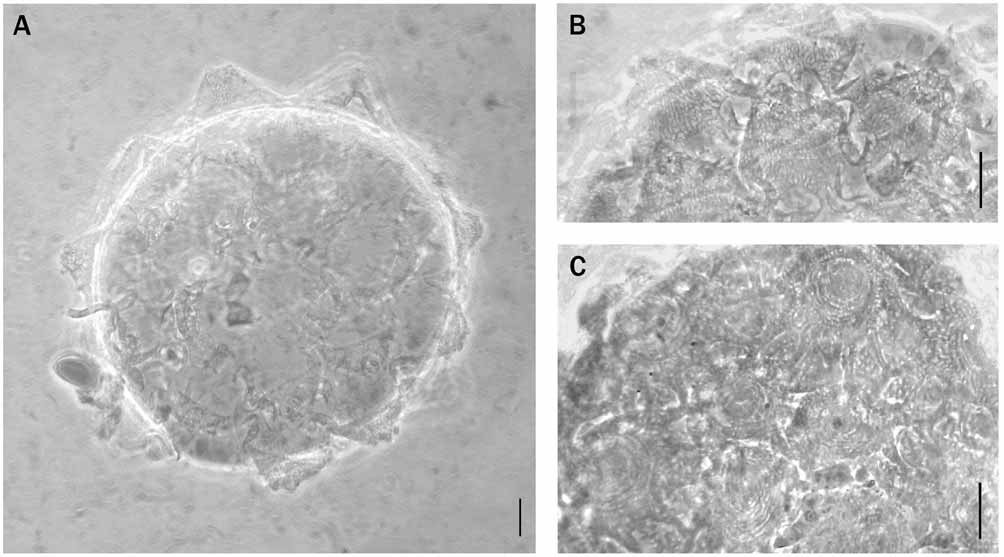
Eggs, laid freely, spherical with truncated conical processes ( Fig. 5 View FIGURE 5 ). In two measured eggs the diameter is 91.0 and 96.0 µm, excluding the processes, and 117.0 and 121.0 µm including them; 11 processes present around the circumference, 24–27 in the hemisphere. Processes 11.0–14.0 µm high, with basal diameter 22.0–23.5 µm. Processes with dense reticular design with elongated meshes having sinuous margins on the basal portion, almost isodiametric on the distal portion ( Fig. 5 View FIGURE 5 B). Egg shell areolated between processes. The large areolae, with thick margins, have a more or less evident thickened central portion with circular light spots forming an irregular reticular design ( Fig. 5 View FIGURE 5 C) (similar to pits in a strawberry).
The paratypes are similar to the holotype in both qualitative and metric characters. Measurements and pt values of selected morphological structures for the smallest and largest examined specimens are given in Table 2 View TABLE 2 .
Etymology: The name ‘ halei ” is in honor of Mr. Gilbert Hale in appreciation of his long-time service as lab assistant for Dr. Diane Nelson. He has produced thousands of slides for the tardigrade inventory of the ATBI, and he is exceptional at finding eggs. Gilbert is a “mighty bear hunter”.
Type depositories: The holotype ( USNM # 1120692) and 3 paratypes ( USNM # 1120693, 1120694, 1120695 (egg)) are deposited in the National Museum of Natural History, Smithsonian Institution, Washington, DC, USA. The remaining paratypes are deposited in the GSMNP collection; the Nelson collection in the Department of Biological Sciences, East Tennessee State University, Johnson City, Tennessee, USA; and the Binda and Pilato collection in the Department of Animal Biology, University of Catania, Catania, Italy.
Differential Diagnosis: Macrobiotus halei sp. nov. is similar to M. vanescens Pilato, Binda & Catanzaro, 1991 , M. priviterae Binda, Pilato, Moncada & Napolitano, 2001 , M. danielisae Pilato, Binda & Lisi, 2006 , M. lorenae Biserov, 1996 , M. gerlachae Pilato, Binda & Lisi, 2004 , M. corgatensis Pilato, Binda & Lisi, 2002 , M. sklodowskae Michalczyk, Kaczmarek & Weglarska, 2006 , M. alekseevi Tumanov, 2005 , M. garynhai Kaczmarek, Michalczyk & Diduszko, 2005 , and M. magdalenae Michalczyk & Kaczmarek, 2006 in having sculptured egg areolae, but it differs from them in some other characters.
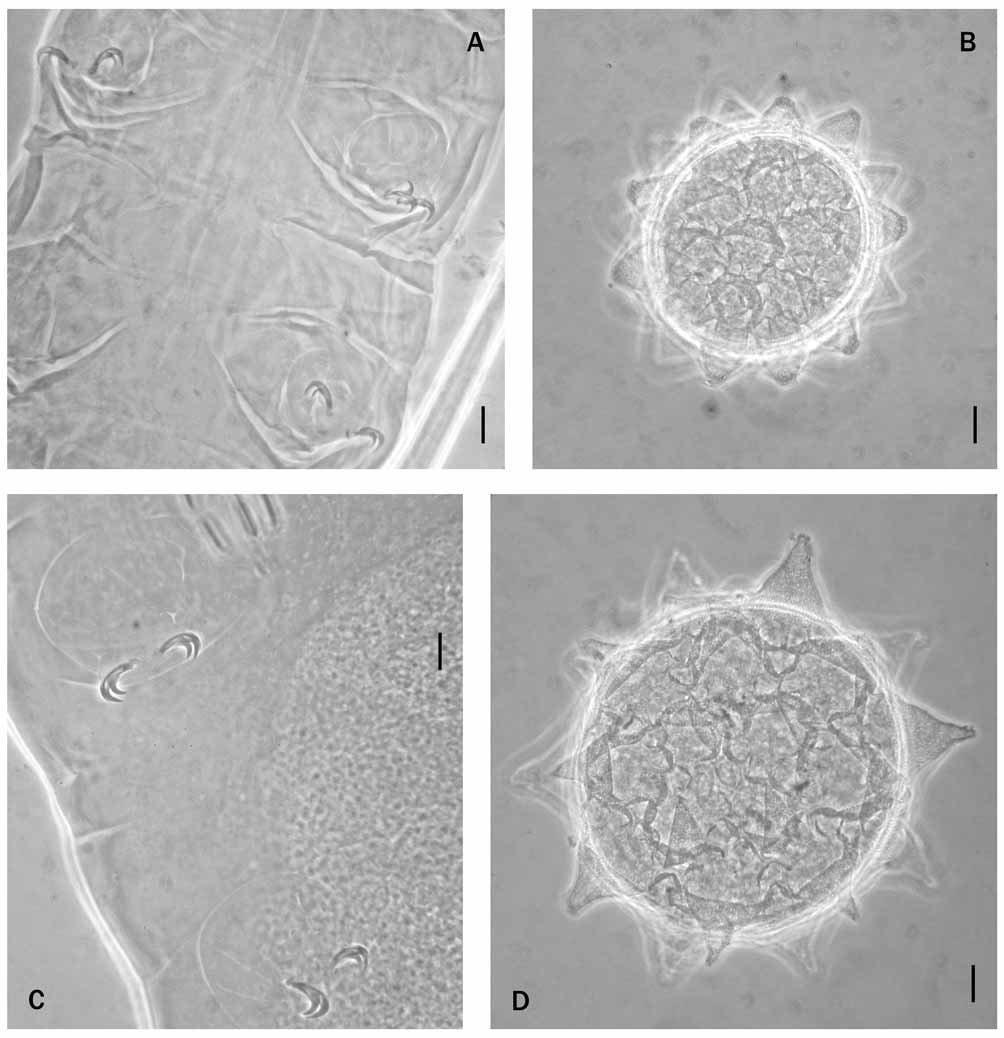
M. halei sp. nov. differs from M. vanescens by having small cuticular tubercles (difficult to see) instead of a very fine punctation; stylet supports inserted on the buccal tube in a more caudal position ( pt = 81.0– 82.7 in M. halei sp. nov., 76.5–77.5 in M. vanescens ); a longer microplacoid ( pt = about 6.0 in M. halei sp. nov., about 4.0 in M. vanescens ) ( Figs. 4 View FIGURE 4 C and 6A); and a different shape of the egg processes, which are slightly shorter (11.0–14.0 µm in M. halei , 16.0–17.0 µm in M. vanescens ) while the basal diameter is similar (22.0–23.5 µm in M. halei sp. nov., 24.0–25.0 µm in M. vanescens ).
M. halei sp. nov. differs from M. priviterae by lacking eyes and by having a sculptured cuticle, a different shape of the claws ( Figs. 4 View FIGURE 4 C and 6B), egg processes with similar height but a larger basal diameter (22.0–23.5 µm in M. halei sp. nov., 13.0–16.3 µm in M. priviterae ) and a more evident reticular design with more elongated meshes.
M. halei sp. nov. differs from M. danielisae by having different cuticular ornamentation (small circular or elongate tubercles in M. halei sp. nov., small polygons of variable and irregular shape in M. danielisae ), by having shorter egg processes (11.0–14 µm in M. halei sp. nov., 17.2 µm in M. danielisae ), of different shape (truncated cones in M. halei sp. nov., cones in M. danielisae ( Figs. 5 View FIGURE 5 and 6 View FIGURE 6. A C), and with the height clearly shorter than the basal diameter while in M. danielisae the height is similar to the basal diameter (the basal diameter is 22.0–23.5 µm in M. halei sp. nov., 17.5–20.0 µm in M. danielisae ).
M. halei sp. nov. differs from M. lorenae by having very small cuticular tubercles and by having a different shape of the egg processes ( Figs. 5 View FIGURE 5 and 6 View FIGURE 6. A D): truncated cones in M. halei sp. nov., and cones terminating with a long, thin, flexible filament in M. lorenae .
M. halei sp. nov. differs from M. gerlachae by having a cuticle ornamented with tubercles, slightly shorter and stouter claws ( Figs. 4 View FIGURE 4 D, E and 7A), and larger eggs, with processes having a larger basal diameter (22.0 – 23.5 µm in M. halei sp. nov., 11.8–14.5 µm in M. gerlachae ) ( Figs. 5 View FIGURE 5 and 7 View FIGURE 7. A B).
M. halei sp. nov. differs from M. corgatensis by lacking eyes, and by having small cuticular tubercles instead of a very fine punctation, stouter claws ( Figs. 4 View FIGURE 4 D, E and 7C), and truncated, conical egg processes ( Figs. 5 View FIGURE 5 and 7 View FIGURE 7. A D) that are shorter (11.0–14.0 µm in M. halei sp. nov., 20.0–25.0 µm in M. corgatensis ).
M. halei sp. nov. differs from M. sklodowskae by lacking eyes, having a sculptured cuticle, a longer and wider buccal tube (the buccal tube width is 14.2–19.1 µm ( pt = 26.5–28.6) in M. halei sp. nov., 7.1–11.9 µm ( pt = 17.0–20.5) in M. sklodowskae ), more slender claws, and less slender egg processes with their basal diameter larger with respect to the process height (height 11.0–14.0 µm and basal diameter 22.0–23.5 µm in M. halei sp. nov.; height 15.2–19.0 µm and basal diameter 29.5 µm in M. sklodowskae ).
M. halei sp. nov. differs from M. alekseevi by having a sculptured cuticle, longer stylet supports inserted on the buccal tube in a more caudal position ( pt = 81.0– 82.7 in M. halei sp. nov., 78.1–81.0 in M. alekseevi ), a wider buccal tube ( pt = 26.5–28.6 in M. halei sp. nov., 16.6–20.3 in M. alekseevi ) and differently-shaped egg processes (in M. halei sp. nov. they are truncated cones without a terminal cap-like vesicular structure).
M. halei sp. nov. differs from M. garynhai by having cuticular tubercles and lacking elliptical pores, and by having egg processes that are truncated cones without a cap-like terminal portion, and by the egg shell areolae with a thickened central portion with circular light spots forming an irregular reticular design).
M. halei sp. nov. differs from M. magdalenae by lacking eyes, having a sculptured cuticle, and by the shape of the egg processes (truncated cones in M. halei sp. nov., cones with elongate terminal portion in M. magdalenae ).
Comments: Besides the holotype and paratypes used for the description of the species in this paper, additional specimens of Macrobiotus halei sp. nov. have been identified within the park. The total is now 361 specimens, including 13 eggs, although we expect to find many more as we sort through additional slides of the M. richtersi group. So far, all M. halei sp. nov. specimens have been found in mosses and lichens on trees and rocks and also frequently in soil and leaf litter. None have been found in our aquatic samples.
TABLE 2. Macrobiotus halei sp. nov. metric characters (all values in µm, pt ratios in brackets).
| smallest specimen | largest specimen | |
|---|---|---|
| Body length | 405.0 | 630.0 |
| Buccal tube length | 53.5 | 66.8 |
| Buccal tube width | 14.2 [26.5] | 19.1 [28.6] |
| Stylet supports | 43.6 [81.5] | 54.4 [81.5] |
| Placoid row | 36.9 [69.0] | 49.6 [74.3] |
| Macroplacoid row | 27.8 [52.0] | 37.0 [55.4] |
| First macroplacoid | 9.2 [17.2] | 12.4 [18.6] |
| Second macroplacoid | 6.4 [12.0] | 8.8 [13.2] |
| Third macroplacoid | 9.8 [18.3] | 12.7 [19.0] |
| Microplacoid | 3.4 [6.4] | 4.0 [6.0] |
| Internal claw II | 10.9 [20.4] | 14.1 [21.1] |
| External claw II | 12.2 [22.8] | 15.5 [23.2] |
| Internal claw III | 11.6 [21.7] | 14.6 [21.9] |
| External claw III | 12.6 [23.6] | 16.3 [24.4] |
| Anterior claw IV | 13.0 [24.3] | 17.5 [26.2] |
| Posterior claw IV | 15.0 [28.0] | 19.1 [28.6] |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |