Achagua cooperae Matson, 2023
|
publication ID |
https://doi.org/10.11646/zootaxa.5352.4.7 |
|
publication LSID |
lsid:zoobank.org:pub:74DCF84E-60ED-49EA-B5E2-A794A60E4D06 |
|
DOI |
https://doi.org/10.5281/zenodo.8435401 |
|
persistent identifier |
https://treatment.plazi.org/id/2053905A-C10D-464D-A68F-694BF3E7E038 |
|
taxon LSID |
lsid:zoobank.org:act:2053905A-C10D-464D-A68F-694BF3E7E038 |
|
treatment provided by |
Plazi |
|
scientific name |
Achagua cooperae Matson |
| status |
sp. nov. |
Achagua cooperae Matson , n. sp.
( Figs. 3 View FIGURES 1–4 , 7 View FIGURES 5–8 , 10–12 View FIGURES 9–10 View FIGURE 11 View FIGURE 12 )
LSID: 2053905A-C10D-464D-A68F-694BF3E7E038
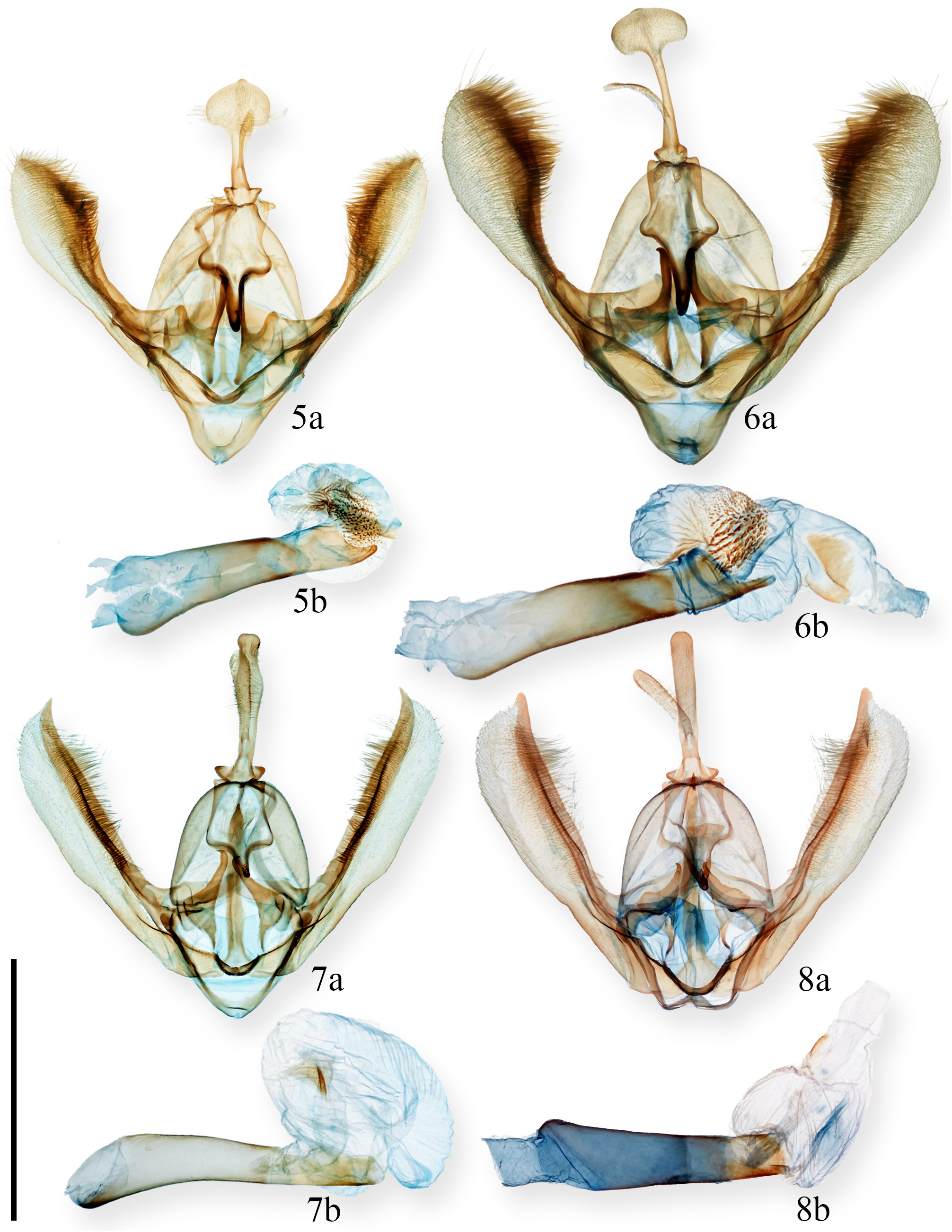
Diagnosis. Achagua cooperae is currently the only member of the genus known from Central America. This species bears a conspicuous antemedial black spot along the forewing inner margin and a weakly gray, discal, reniform spot that are absent in both A. magna and A. obsoleta . The male genitalia of A. cooperae lack a spatulate dorsal process of the uncus, a postmedial digitate protuberance on the costa, and cornuti on the vesica, all of which are found in A. magna and A. obsoleta .
Achagua cooperae is thought to be closely related to A. velata . One noticeable distinction is the thickness of the black terminal band on the hindwing, which tends to be thinner in A. cooperae compared to A. velata . While male A. cooperae share many genitalic similarities with A. velata , the dorsal process of the uncus appears to be knob-like in A. cooperae , whereas in A. velata , it is more uniform in shape ( Figs. 7a, 8a View FIGURES 5–8 ). Achagua cooperae and A. velata may also be separated by their COI barcode (see Molecular characterization).
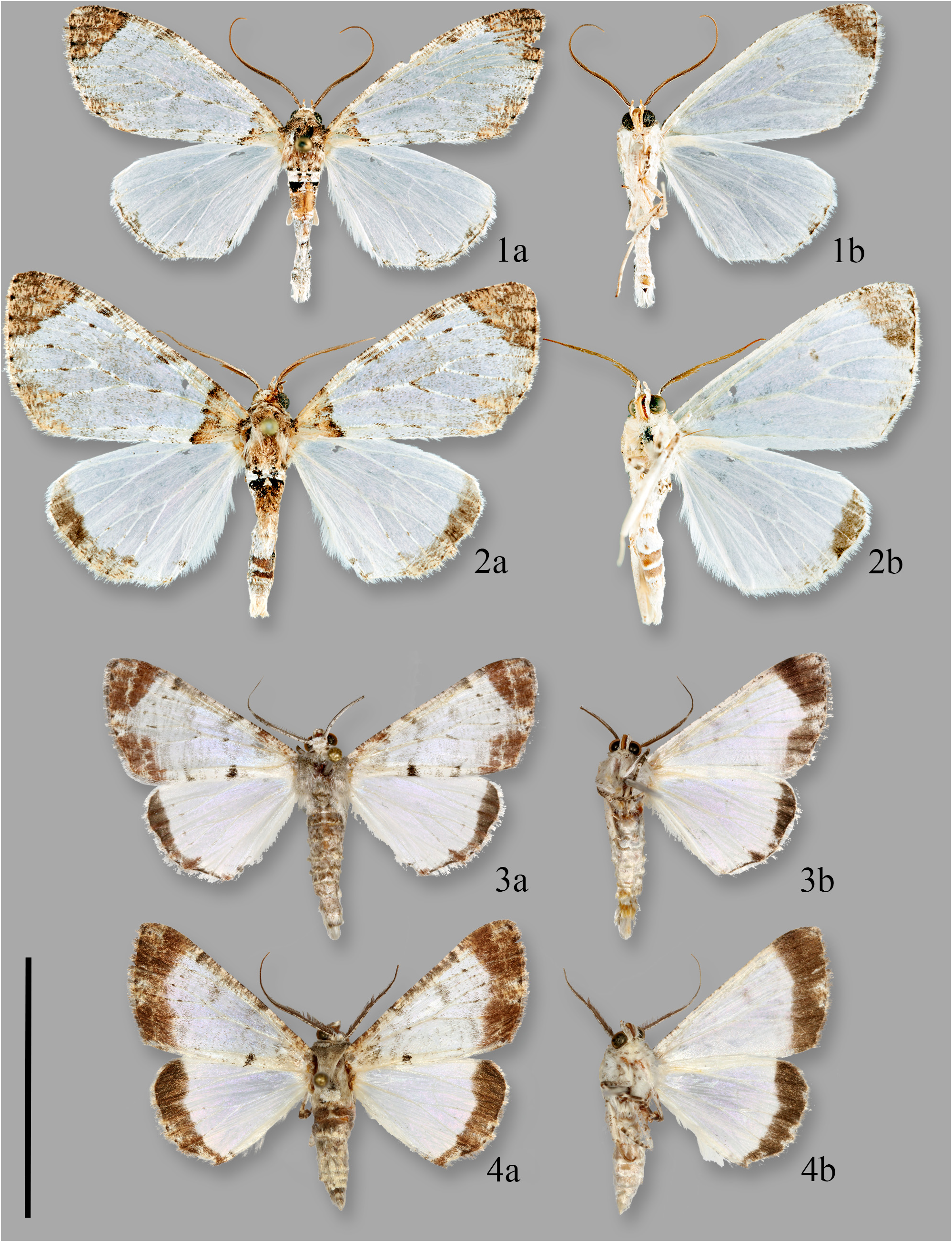
Description. MALE. Forewing length, 17–18 mm (n = 8).
Head. Antenna mostly bipectinate, but with gradually diminishing rami that are absent in distal third of antenna; scales above white and light gray, rami dark gray to black. Vertex white; frons mostly light gray. Labial palpus short, decumbent, 1.5x diameter of eye, light gray and white. Chaetosemata in transverse row; cephalic collar mostly white with few black scales.
Thorax. Patagium, tegula, and mesothorax admixture of gray and white scales. Legs mostly white and mottled with gray; epiphysis well-developed; hind tibia with large hair pencil; tibial spur formula 0–2–4.
Forewing. Pearly-white; widely scattered with inconspicuous light gray scales. Basal and costal areas lightly maculated with gray to brown scales. Subtle, light gray, transverse antemedial line, and weakly gray, discal, reniform spot. Inner margin with antemedial black spot. Terminal area broadly maculated with brown scales and bearing undulating, subterminal pale stripe within. Underside patterned as in upperside but darkened areas more diffuse.
Hindwing. Pearly-white except for blackened terminal area. Underside patterned as in upperside but darkened area more diffuse.
Abdomen. Admixture of gray and white. Third sternite of male abdomen with comb of setae.
Genitalia ( Fig. 7 View FIGURES 5–8 ). Uncus abruptly widened at base; dorsal process larger, gradually enlarging to pronounced swelling at distal third and with knob-like apex; ventral process large and thumb-like. Base of gnathos subquadrangular with upcurved, heavily sclerotized, and pointed apical projection; projection lightly papillated. Valve elongate, quadrate, and large with heavily sclerotized costa; apex strongly falcate. Anellar processes large and triangular, directed inward, and with apical recurved hooks. Juxta with medial, elongate cylindrical process with acuminate apex. Vesica with small medial sclerotized fold; cornuti absent.
FEMALE. Forewing length, 19 mm (n = 2). Outwardly undifferentiated from male.
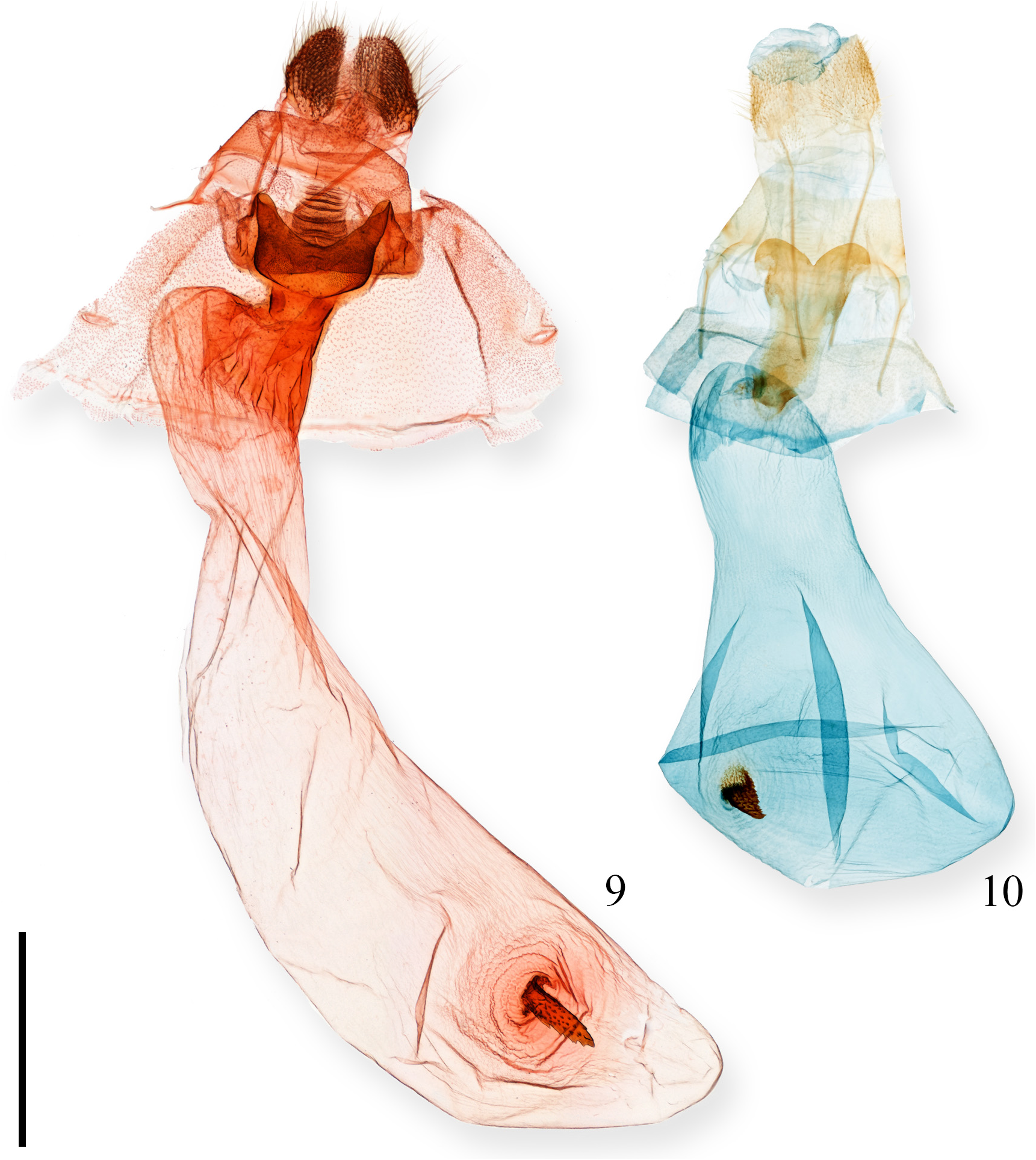
Genitalia ( Fig. 10 View FIGURES 9–10 ). Papillae anales rounded; posterior apophysis 2.5x longer than anterior apophysis. Lamella antevaginalis cordiform; ostium opening into short, lightly sclerotized ductus bursae. Corpus bursae posteriorly narrow, opening into ovoid anterior portion. Signum large and invaginated, appearing somewhat cone-like; surface lightly denticulate.
Type Material.
Holotype
COSTA RICA • ♁; Alajuela, ACG [ Area De Conservación Guanacaste], Rincon Rain Forest , casa de Oscar Albergue ; (10.866°, -85.326°); elev. 725 m; 14 Feb. 2010; R. Franco & H. Cambronero leg. (light trap); Sample IDs : 10-SRNP-105189; Bold Process ID: BLPDQ563 -10; GenBank : HQ934011; USNMENT01771270; USNM.
Paratypes (6♁, ♀)
COSTA RICA • 2♁, ♀; same collection data as holotype; Sample IDs: 10-SRNP-105551, 10-SRNP-105757, 10-SRNP-105758; Bold Process IDs: BLPDQ925-10, BLPDR132-10, BLPDR133-10; GenBank: HQ934144, HQ934347, HQ934348; Genitalia: TAM-2023-289; USNMENT01771269, USNMENT01771271; USN- MENT01771272; USNM • ♁; Alajuela, ACG [Area De Conservación Guanacaste], Rincon Rain Forest, túnel de Oscar Albergue; (10.868°, -85.327°); elev. 708 m; 14 Feb. 2010; S. Rios & F. Quesada leg. (light trap); Sample ID: 10-SRNP-105667; Bold Process ID: BLPDR042-10; GenBank: HQ934257; USNMENT01771273; USNM • 3♁; Puntarenas, 35 km NE of San Vito at Las Alturas Field Station; elev. 4800 ft; [27–29] Apr. 1992; C. Snyder leg. (at light); Genitalia: F.H.R. No. 21326 and TAM-2023-251; AMNH _IZC 00353026 to AMNH _IZC 00353028; AMNH.
Other Material Examined.
MEXICO • ♁; Veracruz, Los Tuxtlas, Sierra Sta. Martha, Arroyo Claro, Neck Point; 19 Mar. 1977; R. Sánchez S. leg.; Genitalia: TAM-2023-315; CNIN • ♀; Veracruz, Est. Biol. de Los Tuxtlas; Alt. 170 m; 01 Apr. 1985; P. Sinaca leg.; Genitalia: TAM-2023-316; 10194; CNIN.
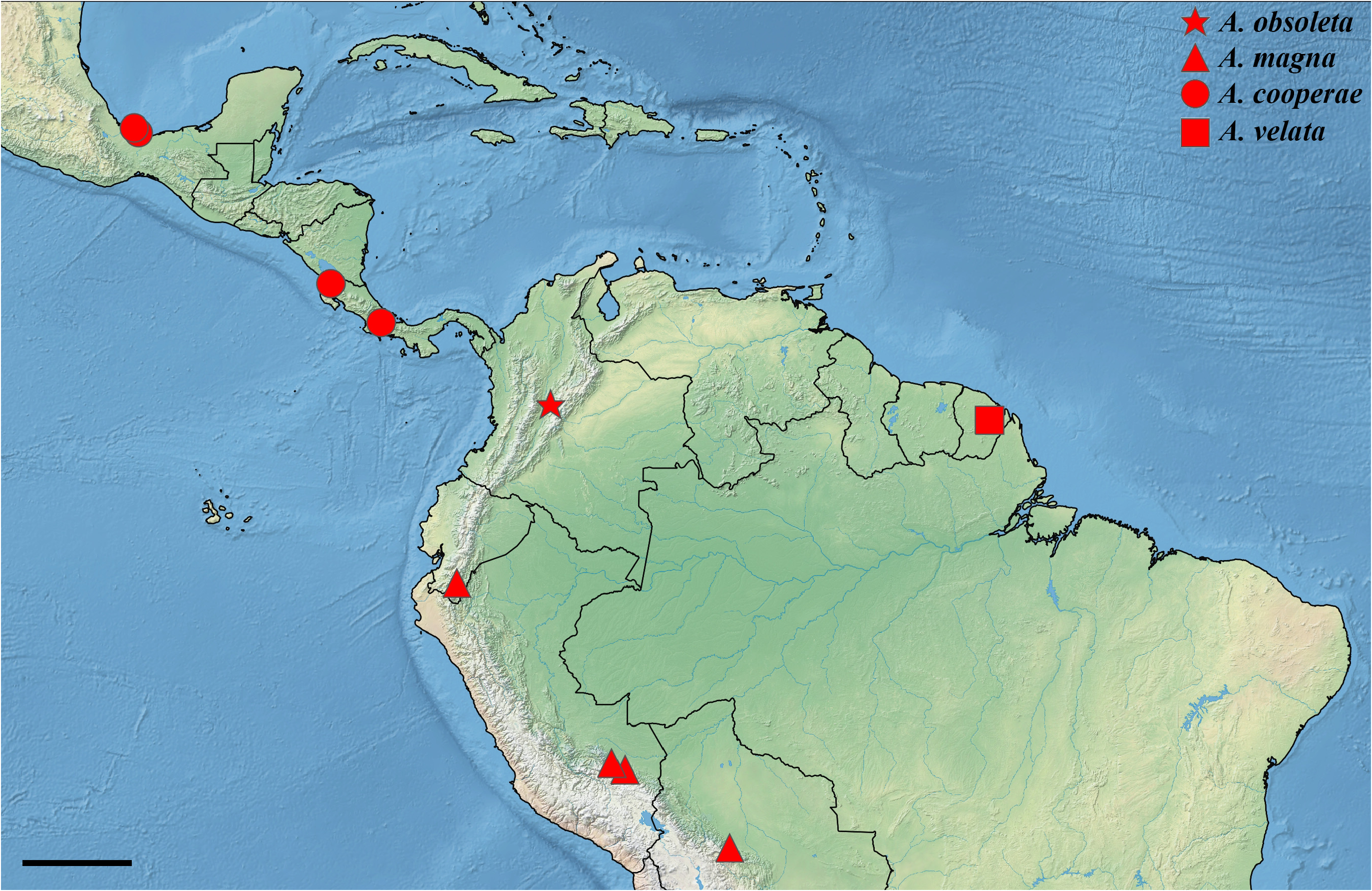
Distribution ( Fig. 11 View FIGURE 11 ). Achagua cooperae is known to inhabit the Isthmian-Pacific and Atlantic moist forests of Costa Rica, but its distribution in other parts of Central America remains uncertain. Notably, two individuals were collected from the Sierra de los Tuxtlas, a remote volcanic range situated along the southeastern coast of the Veracruz Gulf in Mexico. These are tentatively regarded as conspecific (see Remarks).
Biology. The life history of A. cooperae remains unknown. Adult are known to fly from February through April.
Etymology. Achagua cooperae , is named in honor of Loretta Faye Cooper, former Senior Development Officer, and Deputy Director for Advancement at the Smithsonian’s National Museum of Natural History. Loretta’s dedication to museum research is unparalleled, and her tireless efforts to raise awareness and secure funding for the conservation and study of the natural world make her a true champion for both people and the environment. Loretta’s support for the Area de Conservación Guanacaste is particularly noteworthy and greatly appreciated.
Molecular characterization. Achagua cooperae is represented in BOLD by the BIN: BOLD:AAM6724 (n = 5). The pairwise distance to the nearest neighbor, Achagua velata (n = 1, French Guiana), is about 3.8%.
Remarks. I tentatively regard two individuals from the Sierra de los Tuxtlas of Veracruz, Mexico, as A. cooperae . However, I have opted not to include these individuals in the type series. While the genitalia of both sexes in this Mexican population are consistent with those in Costa Rica, the Sierra de los Tuxtlas is a region characterized by biological endemism ( Sánchez-González et al. 2008), and further, this population is disjunct from the remaining known distribution of A. cooperae .
The male phallus depicted in Figure 7b View FIGURES 5–8 may give the impression of a spinate cornutus. However, this is an artifact of the preparation and the structure in question is rather a sclerotized fold.
| USNM |
USA, Washington D.C., National Museum of Natural History, [formerly, United States National Museum] |
| AMNH |
American Museum of Natural History |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Ennominae |
|
Genus |