Trimeresurus wiroti Trutnau, 1981
|
publication ID |
https://doi.org/ 10.11646/zootaxa.1293.1.1 |
|
persistent identifier |
https://treatment.plazi.org/id/039D1618-8544-3804-C219-FA6CFBE02616 |
|
treatment provided by |
Felipe |
|
scientific name |
Trimeresurus wiroti Trutnau, 1981 |
| status |
|
Trimeresurus wiroti Trutnau, 1981
( Figs. 19–22 View FIGURE 19 View FIGURE 20 View FIGURE 21 View FIGURE 22 )
Trimeresurus wiroti Trutnau, 1981: 188 , 139: Fig. 43. Type locality. “Khao Wang Range, at an elevation of between 500 and 1196 m, near villages of [...] Ban Hui Hip and Amphoe Lan Saka” ( Trutnau, 1981, translated from German), Nakhon Si Thammarat Province, Thailand. The type locality was precised by Nutphand et al. (1991: 151) as: “Ban Hui Hip near Khlong Wang Hip, Amphoe Chawang, Changwat Nakhon Si Thammarat (Hui Hip village near Wang Hip canal, Chawang County, Nakhon Si Thammarat Province), approximately 8°30’N, 99°30’E ”, Thailand. Holotype (by monotypy). SMF 69695, juvenile male [not female as stated in Nutphand et al., 1991]. Collected by Wirot Nutphand, 20 June 1979; donated to SMF by Ludwig Trutnau, January 1980.
Chresonymy (not exhaustive) subsequent to the original description only:
Trimeresurus wiroti: Thumwipat & Nutphand (1982: 101) ; Dring et al. (1990: 123, 124); Nutphand (2001: 48, 304–305); this work.
Trimeresurus puniceus wiroti: Cox (1991: 387, 400: Pl. 158).
Trimeresurus puniceus (non C. [raspedocephalus] puniceus Kuhl, 1824 ): Hoge & Romano Hoge (1981: 261); Lim (1982: 23–24, 1991: 25–26); Thumwipat & Nutphand (1982: 100); Tweedie (1983: 141); Welch (1988: 138); Lim (1990: 395); Nutphand et al. (1991: 146, 152); Cox et al. (1991: 20); Jintakune & Lawan (1995: 131); Cox et al. (1998: 24, in part); McDiarmid et al. (1999: 341, in part); Thirakhupt (2000: 168); Orlov et al. (2002b: 354).
Trimeresurus puniceus puniceus: Cox (1991: 386, 400: Pl. 159).
Trimeresurus borneensis (non Atropophis borneensis Peters, 1872 ): Toriba (1992: 71); Golay et al. (1993: 96); David & Vogel (1996: 160 [in part]; Manthey & Grossmann (1997: 409, part.); Chanard et al. (1999: 37); David & Ineich (1999: 281); Gumprecht & Tepedelen (1999: 26); Gumprecht (2001: 28); Nutphand (2001: 48, 306 [as Trimeresurus pumceus ]); Iskandar & Colijn (2001: 157, in part); Orlov et al. (2002a: 189); David et al. (2004: 75); Gumprecht et al. (2004: 30, 162–165); Nabhitabhata et al. (2004: 142).
Material (26 specimens). FEDERATION OF MALAYSIA. West Malaysia. BMNH 1900.7 .18.16 (female), Gunong Murak, State of Perak , 3000 ft; BMNH 1967.2292 (female) , BMNH 1967.2293 (male), between Camps I and II, Gunong Benom, State of Pahang ; BMNH 1970.1917 (male), 22 miles north of Kota Tinggi, State of Johore ; BMNH 1971.1522 (female), Ponti Forest Reserve, State of Johore ; ZMH R05452 (female), “ Johore (Prov.), SMalacca, W. Malaysia ”, now State of Johore .— THAILAND. MTKD D 20743 (female), Trang Province ; MTKD D29153 (male), no precise locality; PSGV 435 (female), no precise locality ; PSGV 6081 , PSGV 6083 (2 females) , PSGV 6082 , PSGV 6084 (2 males) , PSGV 6091 , PSGV 6093 (2 females) , PSGV 6092 , PSGV 774 , PSGV 798 (males), Thung Song , Nakhon Si Thammarat Province ; QSMI 0347 (female) , QSMI 03480349 , QSMI 0389 (3 males), Krabi Province ; SMF 69817 (male) , SMF 74144 (female) , SMF 74145 (male), Thung Song, Nakhon Si Thammarat Province ; SMF 69695 (male; holotype), Hui Hip village near Wang Hip canal, Chawang County, Nakhon Si Thammarat Province .
Taxonomic comments. This species was unintentionally, from a nomenclatural view point, and briefly described by Trutnau (1981) in a book on the husbandry of venomous snakes, on the basis of a single specimen. According to the Code, Art. 73.1.2, this specimen is the holotype by monotypy. It was subsequently indicated by Nutphand et al. (1991) to be specimen SMF 69695. In the text, the author stated that this species, of which five specimens were obtained, was meant to be described by K. Klemmer, then in the “Senckenberg Museum” of Frankfurt ( Germany) on the basis of a single specimen brought back in Germany. Klemmer’s description was never published, but Trutnau (1981) described this lone specimen in details, making his description valid. Trimeresurus wiroti was described in greater details by Nutphand et al. (1991). However, the description of the holotype was erroneous in some aspects. It is briefly redescribed below.
Toriba (1992) addressed the status of Trimeresurus wiroti . On the basis of the contact between the 2 nd SL and the loreal pit, he referred Trimeresurus wiroti to the T. borneensis group, and to the group of T. puniceus . However, due to the limited sample available to him, Toriba (1992) placed Trimeresurus wiroti only tentatively in the synonymy of T. borneensis .
Cox (1991) regarded Trimeresurus wiroti as a subspecies of Trimeresurus puniceus . He summarized in a table the supposed differences between these two subspecies, as follows: (1) head scales stated to be smooth in T. puniceus puniceus vs. slightly keeled in T. p. wiroti ; (2) supraoculars, narrow or divided into erected scales in T. p. puniceus vs. “fragmented” in T. p. wiroti ; and (3) 2 or 3 scale rows between supralabials and suboculars in T. p. puniceus , vs. 1 in T. p. wiroti . Cox (1991) regarded T. p. puniceus as the southern form and T. p. wiroti as the northern form. In a later paper, Nutphand et al. (1991) this author synonymized these two forms. Thumwipat & Nutphand (1982) and Nutphand (2001) also used both names puniceus and wiroti for Thai animals. According to the pictures appearing in these respective publications, it seems that Cox (1991) and Nutphand (2001) merely applied the name puniceus to females and the name wiroti to males of the same species.
On the basis of several morphological differences and of a high genetic distance (14.3 %) between Trimeresurus borneensis from Borneo and of populations from Thailand and West Malaysia ( OTU 5 + OTU 6), we here revalidate at specific level Trimeresurus wiroti Trutnau, 1981 .
Diagnosis. A species of the genus Trimeresurus , endemic to Peninsular Thailand and West Malaysia, characterized by the combination of the following characters: (1) an usually overall dark or very dark pattern, with 22–35 darker crossbands, related to the sex: in males, background colour in various shades of dark greyishbrown, with dark brown irregular dorsolateral blotches, with below an irregular, elongated smaller blotch of same colour; areas between the bloches darker than on the sides of body, heavily powdered with dark and light dots or blotches producing a confused pattern, but not lichenlike; in females, pattern less complex and often as dark or darker than in males, in shades of dark brown with darker subrectangular dorsolateral blotches, without the dotted pattern but with broad darker edges and a wide lighter centre producing a “saddlelike” pattern; males have a more complex pattern, but are as dark as females (2) a distinctly projected and raised snout, strongly obliquely truncated when seen from the side, subrectangular seen from above; (3) internasals projected, strongly spatulate and bilobate, distinctly upturned; (4) usually 21 (rarely 19, 20 or 23) DSR at midbody; (5) 1 st supralabial distinct from nasal; (6) 2 nd supralabials bordering the whole of the anterior margin of the loreal pit; (7) 2 to 4 small and narrow supraoculars, convex or granular and raised; (8) VEN: 159–167 in males and 158–167 in females, SC: 43–56; (9) occipital and temporal scales moderately keeled or smooth in both sexes in adults, usually smooth in juvenile specimens; (10) IL of the first pair not in contact each with the other; and (11) hemipenes short, reaching 9th SC, with spines.
Redescription of the holotype. The specimen on which Trutnau’s (1981) description was based, was identified and redescribed in details by Nutphand et al. (1991). However, some values cited in this latter paper are erroneous. We here redescribe the specimen SMF 69695 as follows (values appearing in Nutphand et al. [1991] are placed in square brackets).
Juvenile male [not female]; SVL 253 mm [285 mm], TaL 49 mm [45 mm]; ratio TaL/ TL 0.162. DSR: 23–21–15 [17–21–13], smooth throughout [“dorsal scales strongly keeled, weakly on sides”]. Body: 166 VEN (plus 2 preventrals) [177], SC: 56 [51], all paired. Head: 9/10 SL [9/9], 10 Cep, 13/13 IL [8/8]. Other characters are typical of the species, as defined below.
Description and variation. We did not identify morphological variation correlated to the geographical origin among our sample of 26 specimens.
The maximal total length known is 889 mm (SVL 762 mm, TaL 127 mm) for a female (PSGV 435). The largest known male is 655 mm long (SVL 553 mm, TaL 102 mm; PSGV 774). In our sample of 26 specimens, there is no noticeable difference in maximal sizes of males and females. This species may reach a size similar to Trimeresurus puniceus and Trimeresurus borneensis , although available specimens are usually 400–600 mm long.
Body relatively slender in males but stout in females. Triangular head elongated, average, amounting (in adults above SVL 400 mm) for 5.1–6.5 % of SVL (x = 5.6 %) in males, 5.6–6.9 % of SVL (x = 6.3 %) in females, wide at its base, flattened, thin in males, thick and swollen in females when seen from the side. Snout distinctly projected and raised anteriorly, strongly obliquely trunctated when seen from the side, with a distinct canthus rostralis, flattened and rectangular when seen from above, with strongly spatulate and bilobated internasal scales; the snout is rather long, amounting (in adults) for 23.5–28.9 % (x = 26.1 %) of HL in males and 25.6–29.7 % (x = 27.9 %) of HL in females, or 1.8–2.5 (x = 2.2) times in males and 2.3–2.6 (x = 2.4) times as long as diameter of eye. Eye average, amounting for 0.9–1.2 (x = 1.0) times in males and 0.8–0.9 (x = 0.8) times in females of the distance eye–lip. Tail tapering progressively and strongly prehensile. Ratio TaL/TL: 0.129 –0.167, with a strong sexual dimorphism (see below).
DSR: 21–23 (25 or 29)—(19 or 20)21 (23)—13–15(16), smooth or weakly keeled in both sexes, smooth on 1 st DSR.
In our samples of 26 specimens, the number of MSR is as follows: 19 (1/26), 20 (2/ 26), 21 (20/26) and 23 (3/26).
VEN: 158–167 (plus 1–2 preventrals); SC: 43–51, all paired; anal shield entire.
Rostral not visible from above, about 1.3–1.6 times broader than high, triangular; nasals subrectangular, entire; usually 2 (48/56 occurrences), rarely 1 (8/56) internasals on each side, enlarged, strongly projected, spatulate and strongly bilobate, raised and distinctly upturned; internasals or group of internasals separated by 1 (in 6/ 26 specimens) or 2 (in 20/26) small scales; 3–5 canthal scales, slightly larger than adjacent snout scales, bordering the canthus rostralis; 2 elongate loreal scales, subrectangular, between upper preocular and nasal scales in all examined specimens; two elongated upper preoculars above the loreal pit; lower preocular forming the lower margin of loreal pit; 2–3 small postoculars; 2–4 small, narrow supraoculars on each side (2: 23/52 occurrences, 3: 23/52, 4: 6/52; total number: 4–8; x = 5.3, s = 1.3), convex (12/52 occurrences) or raised (40/52) but not extending out of the margin of the head, in total 2.2–2.8 (x = 2.5) times as long as wide, 0.5–0.7 (x = 0.6) time as wide as the group of internasals, irregularly bordered on their inner margins by the upper head scales; 5–8 (x = 6.5, s = 0.8) slightly enlarged scales on upper snout surface on a line between the scale separating the internasals and a line connecting the anterior margins of eyes, smooth, juxtaposed, irregular in shape (most common numbers: 5 in 12/26 and 6 in 8/ 26 specimens); 9–12 (x = 10.5, s = 0.8) cephalic scales on a line between supraoculars (10: 11/26; 11: 12/26), small, smooth, flat and juxtaposed; occipital scales larger than cephalic scales, moderately keeled in both males and females; temporal scales small, subequal, in 2 or 3 rows, usually moderately (10/ 13 specimens) keeled or smooth (3/13) in males, moderately (9/13) or smooth (4/13) in females; one thin, elongated, crescentlike subocular; 9–11 SL (x = 10.1, s = 0.7) (9: 9/52 occurrences; 10: 32/52; 11: 9/52; 12: 2/52), with most often the combinations 10–10 SL); 1 st SL, rather short, always separated from nasal; 2 nd SL tall, entirely bordering the anterior margin of the loreal pit, separated from nasal by 1 or usually 2 small scales; 3 rd SL longest and highest, 1.3–1.8 times as long as high (x = 1.6), separated from the subocular by usually 1 (38/52 occurences) or 2 (14/52) scales, without clear sexual dimorphism; 4 th SL as long as high, 0.8–1.2 (x = 0.9) time as low as 3 rd SL, separated from the subocular by 1 (19/52 occurrences) or 2 (33/52) scales on each side, with a sexual dimorphism (2 scales in 10/26 occurrences in males 22/26 occurrences in females); 5 th and posterior SL smaller than 4 th one, 5 th SL separated from subocular by 1 (in 2/52 occurrences), 2 (46/52) or 3 (4/ 52) scale rows of similar size, without clear sexual dimorphism, although the presence of 1 row occurs only in males and 3 rows only in females; 10–15 (usually 11–13) IL (10: 6/50 occurrences; 11: 7/50; 12: 18/50; 13: 14/50; 14: 4/50; 15: 1/50); scales of the 1 st pair not longitudinally in contact, being divided into two scales; first two pairs in contact with anterior chinshields; 8–9 rows of smooth gular scales; throat shields irregularly arranged.
In life, the coloration pattern of Trimeresurus wiroti is also quite variable and related to the sex, but to a lesser extant than in previous species.
In males, the body background colour is olivebrown, dark greyishbrown or dark yellowishgrey, with about 22–35 large, dorsolateral dark brown, dark reddishbrown, dark purple brown or dark greyishbrown blotches; between the blotches, the background colour is often darker than on the sides of body, with scales heavily powdered with dark grey or dark brown dots or marked with small, irregular, dark blotches and with lighter irregular dots giving a confused pattern but not “ashy” like in T. puniceus ; the large dorsolateral blotches are irregular in shape, subrectangular or more or less constricted in their middle, reaching down the 6 th or 7 th DSR, with below an irregular, elongated blotches of same colour separated from the main blotch by a lighter coloration; the centre of the upper dorsolateral blotches is somewhat lighter, their edge more or less darker, often with white or cream dots, producing the “saddlelike” pattern depicted in Hoge & Romano (1974); the blotches are usually separated at the level of their upper part; blotches of both sides of body may be either fused or alternate, with all intermediates between perfect opposition and total alternation; on the tips of ventrals and on the 1 st DSR, a series of dark, elongated ventrolateral blotches, more or less irregular. The tail surface is usually of the same colour than the dorsolateral blotches, usually dark brown or dark reddishbrown, with pale greyishbrown or yellowishbrown rings, often edged with cream; the last third or so of the tail is entirely paler reddishbrown or yellowishbrown.
In females, as observed in T. borneensis , the pattern is less complex but not necessarily lighter, sometimes even darker; the body is dark yellowochre, greyishbrown, reddishbrown or dark greenishbrown, with large, subrectangular, not constricted, dark brown, dark yellowish, pinkish, greyish or reddishbrown or dark grey blotches, in same number than in males; these blotches are usually not vertically divided and reach down the 2 nd or 3 rd DSR; they are usually separated one from the other on their sides, with both broad darker edges and a wide lighter centre, producing “saddlelike” pattern that Hoge & Romano (1974: 158) credited to T. puniceus ; blotches of both sides of body may be either fused or alternate, with all intermediates; overall pattern of females may be somewhat similar to males, although with a paler background. The tail surface is as described in males, dark brown, chocolate or dark reddishbrown, with pale greyishbrown or reddishbrown rings, the last part being entirely reddishbrown or yellowishbrown. Males can be distinguished from females by their more complex pattern but are not necessarily darker than females. Cox (1991) identified males as T. puniceus wiroti and females, with their more reddishbrown pattern as T. puniceus puniceus .
In both sexes, the dorsal head surface and temporal regions are of the same dark colours as the dorsolateral blotches; in males, the sides of the head and supralabials are often irregularly colored, with a complex pattern of light markings on a dark background, with a mixing of different pale and dark hues and scales heavily powdered with dark minute dots; the lower margin of supralabials is sometimes much lighter or cream; in females, the sides of the head are heavily powdered with minute black dots; a more or less distinct, more or less wide orangebrown, pale reddishbrown or yellowishochre postocular streak extending on upper temporals from the eye to the corner of the mouth in widening progressively before vanishing in general body colour; the postocular streak is edged below with a dark streak of same colour than upper head surface, itself sometimes but not necessarily edged with a short area of paler hue below at the limit of the throat and the edge of the supralabials. Eyes are golden, yellowishbrown or brownishgrey, more or less dark.
Venter is much darker than dorsal background coloration, usually dark reddishbrown or dark ochrebrown, irregular in colour and mottled with several hues of brown, powdered with numerous minute black dots and small white markings; tips of ventrals with irregular elongated blotches of same colour than dorsolateral blotches intermixed with white blotches, making a very irregular, broken ventrolateral stripe. The chin and throat are paler than upper head surface, with numerous black dots and cream or pale brown irregular markings.
In juveniles, the colour and pattern are basically identical, but background colour of males and females is lighter than in adults, most often in the hues of ochre, pale yellowishbrown, greyishbrown or greenishgreen, with darker dorsolateral blotches, whereas males show a more contrasted pattern. The posterior part of the tail is yellow.
In alcohol, T. wiroti shows a wide range of brown, greyishbrown, and so on, producing the patterns described above, but much less conspicuous and rather darkened. Females tend to be much darker than males, with a more subdued pattern, whereas it remains more complex with irregular hues in males.
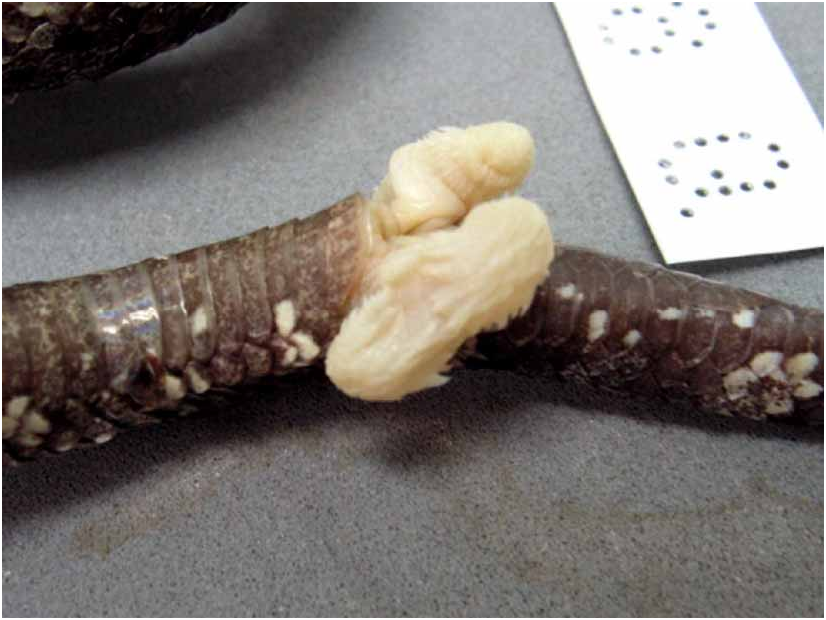
Hemipenes ( Fig. 19 View FIGURE 19 ). According to Jintakune & Chanhome (1995) and examined specimens, the hemipenes are short, bilobate, reaching 9 th or 10 th SC, forked opposite 3 rd or 4 th SC. Most of the organ is covered with calyces distally; many short spines and one or two strongly enlarged spines proximally to the forking point.
Comparison with other species. Trimeresurus wiroti belongs to the Trimeresurus borneensis group and differs from T. puniceus and T. cf. puniceus by the general characters given above to separate these groups.
T. wiroti differs from other species of the Trimeresurus borneensis group by characters given in Tables 7–8. From T. borneensis , T. wiroti differs mostly by the combination of the following characters: (1) ratio TaL/TL in males, 0.156 –0.167 (x = 0.163, n = 13) in T. wiroti vs. 0.165 –0.189 (x = 0.173, n = 13) in T. borneensis (U = 5.5, P <0.001); (2) a higher number of VEN in females, 158–164 (x = 161.0, n = 13) in T. wiroti vs. 149–163 (x = 155.8, n = 15) in T. borneensis (U = 22.0, P <0.001); (3) supraocular scales small and often raised, in 40/52 occurrences, whereas they are larger and all flat in T. borneensis (56/ 56 occurrences); (4) a higher number of supraoculars in T. wiroti : the total of supraoculars on both sides varies from 4 to 8 in T. wiroti (x = 5.3, n = 52) vs. 2 to 6 (x = 3.2, n = 56) in T. borneensis (U = 84.5, P <0.001); and (5) a much darker pattern in adult females of T. wiroti than in T. borneensis . The most conspicuous morphological difference between T. wiroti and T. borneensis appears in females. Pholidosis and colouration of the males are quite similar, what may explain why Toriba (1992), who had only two males at hand, regarded these two forms as conspecific. Females of T. borneensis are much lighter than those of T. wiroti .
From Trimeresurus brongersmai , T. wiroti is separated by the following combination of characters: (1) a larger size in T. wiroti , with, in females, a TL max of 889 mm vs. 441 mm in T. bronge rsmai; (2) the number of MSR, 19–23 (x = 21.1, s = 0.9) in T. wiroti vs. 19–21 (x = 19.6, s = 0.9) in T. brongersmai ; (3) the shape of supraoculars, strongly erect, directed upwards and outwards in T. brongersmai , convex or raised but not “spiny” nor extending out of the head margin in T. wiroti ; (4) a higher number of ventral scales in both sexes in T. wiroti than in T. brongersmai , without overlap (see Table 7); (5) a higher number of subcaudal scales in both sexes in T. wiroti than in T. brongersmai , with slight overlap (see Table 7) (in males: U = 0.5, P <0.001; in females: U = 1.5, P <0.001); (6) a higher number of Cep in T. brongersmai (11–15; x = 13.0, s = 1.3) than in T. wiroti (9–12; x = 10.5, s = 0.8) (U = 2, P <0.001); and (7) occipital and temporal scales smooth or weakly keeled in T. wiroti , strongly keeled in T. brongersmai .
Lastly, the differences between Trimeresurus wiroti and Trimeresurus andalasensis spec. nov. are given below in the account of this latter species.
Sexual dimorphism. It is strongly marked in the relative length of the tail, in the number of ventrals and in the pattern:
(1) Difference in the ratio TaL/TL:
males: 0.156 –0.167 (x = 0.163, s = 0.003); females: 0.129 –0.143 (x = 0.137, s = 0.005)
(2) Difference in the average numbers of subcaudals, with a small overlap of values:
49–56 (x = 53.3, s = 2.6) in males vs. 43–51 (x = 47.8, s = 3.3) in females.
(3) Difference in the pattern. As observed in Trimeresurus borneensis , males show a more complex and contrasted pattern than females, but the latter ones are usually darker than males in overall pattern.
Range ( Fig. 4 View FIGURE 4 ). FEDERATION OF MALAYSIA. West Malaysia. This species is known from the states of Johore, Pahang, Perak, and Selangor ( Lim, 1963, 1967; Lim & Bakar bin Ibrahim, 1970; Grandison, 1978; Tweedie, 1983; examined specimens). THAILAND. Trimeresurus wiroti has been recorded only in hilly areas from southern Peninsular Thailand, from the provinces of Krabi, Nakhon Si Thammarat, Narathiwat and Trang (Cox, 1991; Nabhitabhata et al., 2004; examined specimens).
Biology. According to Lim & Bakar bin Ibrahim (1970), Trutnau (1981), Cox (1991), Cox et al. (1998), Trimeresurus wiroti is an inhabitant of primary wet lowland and hill forests. It has been recorded in the forest canopy at more than 20 meters above the ground. It is known to take mammals, birds and frogs. This species is oviparous, laying 7– 12 eggs which need about 6–8 weeks to hatch (Cox, 1991; H. Bungert, pers. comm., October 2005).
The first specimen ever recorded of Trimeresurus wiroti seems to be BMNH 1900.7.18.16, obtained in West Malaysia, State of Perak, by Mr. L. Wray (see Boulenger, 1912). The holotype of Trimeresurus wiroti was collected in June 1979 (see Nutphand et al., 1991), but the species had previously been recorded, as Trimeresurus puniceus , from Narathiwat Province ( Nutphand et al. (1991).
Comments. Trimeresurus wiroti has been more depicted alive in the literature than any other species of the T. puniceus complex. Colour pictures appear in Trutnau (1981: 139: Fig. 43), Thumwipat & Nutphand (1982: 140: Fig. 67 [as Trimeresurus puniceus , in fact a female specimen], 141: Fig. 70 [as Trimeresurus wiroti , in fact a male specimen]); Lim (1982: 24: Fig. 24 View FIGURE 24 , 1990: 396: Fig. 10 View FIGURE 10 , 1991: 26: Fig. 29 View FIGURE 29 ); Cox (1991: 400: Pls. 158–159), Jintakune & Chanhome (1995: 133: Figs. 197–199), Manthey & Grossmann (1997: 408: Fig. 314), Cox et al. (1998: 24), Nutphand (2001: 304–305 [as Trimeresurus wiroti , in fact male specimen]; 306 [as Trimeresurus puniceus , in fact female]) and Gumprecht et al. (2004: 162: Figs. I–I, 163: Figs. I–IV, 164: Figs. I–II, 165: Figs. I–V). Ink drawings of the head appeared in Nutphand et al; (1991: 149: Fig. 1 View FIGURE 1 ).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Trimeresurus wiroti Trutnau, 1981
| David, Patrick, Vogel, Gernot, Vijayakumar, S. P. & Vidal, Nicolas 2006 |
Trimeresurus borneensis
| David, P. & Cox, M. J. & Pauwels, O. S. G. & Chanhome, L. & Thirakhupt, K. 2004: 75 |
| Gumprecht, A. & Tillack, F. & Orlov, N. & Captain, A. & Ryabov, S. 2004: 30 |
| Nabhitabhata, J. & Chuaynkern, Y. 2004: 142 |
| Orlov N. & Ananjeva, N. & Barabanov, A. & Ryabov, S. & Khalikov, R. 2002: 189 |
| Gumprecht, A. 2001: 28 |
| Nutphand, W. 2001: 48 |
| Iskandar, D. T. & Colijn, E. 2001: 157 |
| David, P. & Ineich, I. 1999: 281 |
| Gumprecht, A. & Tepedelen, T. 1999: 26 |
| Manthey, U. & Grossmann, W. 1997: 409 |
| David, P. & Vogel, G. 1996: 160 |
| Golay, P. & Smith, H. M. & Broadley, D. G. & Dixon, J. R. & McCarthy, C. J. & Rage, J. - C. & Schatti, B. & Toriba, M. 1993: 96 |
| Toriba, M. 1992: 71 |
Trimeresurus wiroti: Thumwipat & Nutphand (1982: 101)
| Nutphand, W. 2001: 48 |
| Dring, J. C. M. & McCarthy, C. J. & Whitten, A. J. 1990: 123 |
| Thumwipat, B. & Nutphand, W. 1982: ) |
Trimeresurus wiroti
| Nutphand, W. & Cox, M. J. & Trutnau, L. & Smith, H. M. 1991: 151 |
| Trutnau, L. 1981: 188 |
Trimeresurus puniceus
| Orlov, N. & Ananjeva, N. & Khalikov, R. 2002: 354 |
| Thirakhupt, K 2000: 168 |
| McDiarmid, R. W. & Campbell, J. A. & Toure, T'S 1999: 341 |
| Cox, M. J. & van Dijk, P. P. & Nabhitabhata, J. & Thirakhupt, K. 1998: 24 |
| Lim, B. L. 1991: 25 |
| Nutphand, W. & Cox, M. J. & Trutnau, L. & Smith, H. M. 1991: 146 |
| Cox, M. J. & Drew, D. & Chiszar, D. & Smith, H. M. 1991: 20 |
| Lim, B. L. 1990: 395 |
| Welch, K. R. G. 1988: 138 |
| Tweedie, M. W. F. 1983: 141 |
| Lim, B. L. 1982: 23 |
| Thumwipat, B. & Nutphand, W. 1982: 100 |
| Hoge, A. R. & Romano Hoge, S. A. R. W. L. 1981: 261 |