Coronellina atlantica, Souto, Javier, Reverter-Gil, Oscar & Ostrovsky, Andrew N., 2014
|
publication ID |
https://doi.org/10.11646/zootaxa.3795.2.3 |
|
publication LSID |
lsid:zoobank.org:pub:6DE4B4A5-9369-4F9A-9F1A-C61EC4528F3A |
|
DOI |
https://doi.org/10.5281/zenodo.6144302 |
|
persistent identifier |
https://treatment.plazi.org/id/039D879A-FFA0-4C61-FF16-F9BCFA32FB74 |
|
treatment provided by |
Plazi |
|
scientific name |
Coronellina atlantica |
| status |
sp. nov. |
Coronellina atlantica n. sp.
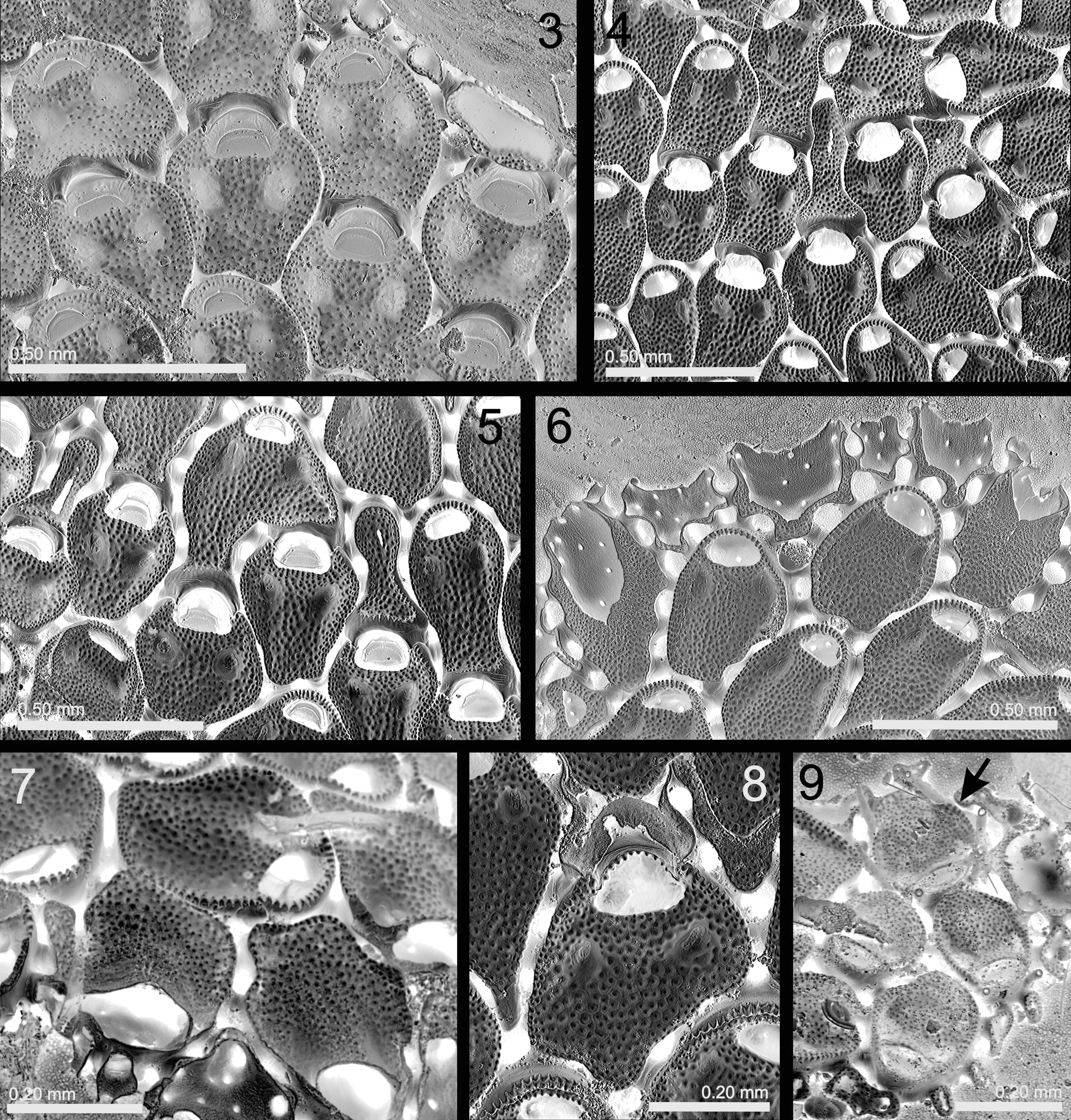
( Figs 3–9 View FIGURES 3 – 9 ; Table 1 View TABLE 1 )
Material examined. Holotype: MNCN –25.03/3863, 32º38’30.67’’ N, 16º49’47.49’’ W, 18 m, April 2011, on rhodoliths. Paratypes: MNCN –25.03/3859, 3868, NHMUK 2014.1.16.1: data as in holotype.
Etymology. From the Atlantic origin of the samples, in contradistinction to the Mediterranean distribution of the type species of the genus.
Description. Colony encrusting, multiserial, unilaminar. Zooids generally arranged in alternating series, sometimes irregularly disposed; mostly disjunct, separated by a wide slit, but sometimes nearly contiguous with only a deep groove between them. Each zooid linked to its neighbors by 10–14 short tubules, each formed from a basal pore-chamber developing on all vertical cystid walls including the proximal one; each zooid thus connected to a neighbor by 2–3 (sometimes 1 or even 4) tubules. Occasionally a bifurcate tubule is linked to two proximal zooids. Autozooids generally oval, but frequently having an irregular shape, adapting to the substratum and neighboring zooids. Cystid outline distally rounded whereas it is truncate or, more frequently, irregular proximally. Frontal wall membranous, underlain by a flat granular cryptocyst with raised crenellate lateral rim (often nondeveloped proximally). Cryptocyst occupying about 80% of frontal side, being depressed in its distal third and slightly raised towards the opesia. Two large, non-perforate depressions ( 0.04–0.06 mm long, 0.020–0.04 mm wide) proximal to opesia; these shallow in newly formed zooids, becoming deeper with increasing calcification. Opesia transversely elliptical or semicircular, widest proximally; its proximal edge straight or slightly concave, having no laminar lip. Operculum semicircular, with a marginal sclerite, occupying most of the opesia.
Cystid basal wall not in direct contact with substratum but supported on small basal rootlets (seen as tiny pits in developing zooids, Fig. 6 View FIGURES 3 – 9 ), comprising short outgrowths attached to the substratum.
Ovicell immersed, acleithral, with ooecium formed by the distal autozooid or, sometimes, kenozooid. Ooecium vestigial, with proximal gymnocystal edge and distal cryptocystal area. Brood sac (removed during bleaching) placed below operculum of maternal zooid, which has a larger, slightly trifoliate, opesia. Operculum of maternal zooids larger than in non-fertile autozooids. Up to two ooecia formed by a single distal autozooid seen in several specimens ( Fig. 5 View FIGURES 3 – 9 ).
Abortive kenozooids sometimes present; these irregularly shaped, with granular cryptocyst and a small central or distal slit in the cryptocyst.
Ancestrula autozooidal, with smaller opesia and six mural spines: two distal (oral), two lateral and two proximal. At least one periancestrular zooid with two distal oral spines.
SD, Standard deviation; N, number of measurements.
Remarks. Coronellina atlantica n. sp. resembles both C. fagei ( Gautier, 1962) and several species of the genus Mollia . As in these species, C. atlantica has more-or-less oval zooids that are disjunct and linked to neighbors by tubules, an extensive cryptocyst leaving an opesia larger than the operculum, and crenulate zooidal margins without spines, except in the ancestrula and periancestrular zooids. The structure of the immersed ovicells is also similar. In both genera, colonies are attached to the substratum by basal rootlets outgrowing from the basal wall.
Coronellina atlantica differs from Mollia View in CoL mainly by the presence of the two depressions proximal to the opesia. Coronellina fagei View in CoL , the only species of the genus up to now, was originally described from the Mediterranean and recently redescribed in our previous work ( Souto et al. 2010a). However, the ovicells of this species have been previously described by Prenant & Bobin (1966) and Souto et al. (2010a, p.42) as “independent, hyperstomial, with recessed frontal surface flat, granular, sloping towards the distal zooid”. In fact, this species possesses the same ovicell structure as C. atlantica – immersed with a vestigial ooecium and brooding cavity beneath the maternal opeculum. In contrast with C. atlantica , however, the vestigial ooecium of C. fagei View in CoL is formed by a wide distal kenozooid with a round outline (in frontal view). This kenozooid mimics a conventional (hyperstomial) ovicell, and this is a reason why the former was confused with the latter. In contrast, the vestigial ooecium of the immersed ovicells of C. atlantica is formed by a distal autozooid, although sometimes by an irregularly shaped distal kenozooid ( Figs 4, 5 View FIGURES 3 – 9 ).
The autozooids of C. fagei View in CoL tend to be more oval (autozooidal length/autozooidal width = 1.37–1.45), while those of C. atlantica are more elongated (autozooidal length/autozooidal width = 1.54); however, we admit that this difference is negligible and could be connected with the substratum.
Another important difference between the two species is the presence of a pair of opesiules in C. fagei View in CoL , while C. atlantica has, in the same position, a pair of large opesiule-like depressions. Also the development of the cryptocyst in newly budded autozooids differs. In both species, the cryptocyst grows from the proximal margin in a distal direction. In C. fagei View in CoL (see Souto et al. 2010a, fig. 11) the growth of the cryptocyst results first in a wide trifoliolate ‘primordial’ opesia, with its proximal rim extending distally in a kind of calcified tongue. This tonguelike lip of calcification finally fuses with two lateral cryptocystal outgrowths, forming the proximal lip of the final opesia, leaving two lateral holes (opesiules) in the cryptocyst. The proximal lip projects slightly, having a pair of lateral notches through which the opercular muscles should pass.
In C. atlantica a median calcified tongue is not formed. Instead, the centripetally growing cryptocyst fuses along the midline of the zooid, temporarily leaving a medial suture ( Fig. 6 View FIGURES 3 – 9 ). Further, the growing margin of the cryptocyst forms the proximal edge of the opesia, lacking a medial lip and notches. Proximal to the opesia there are two opesiule-like depressions in the cryptocyst, which become deeper and more conspicuous as calcification advances. However, they never acquire a perforation during cryptocyst formation and cannot constitute opesiules through which parietal muscles pass. We have not been able to study live material, but while in C. fagei View in CoL the parietal muscles obviously go through the opesiules, in C. atlantica these must be located within the opesia.
Several groups of anascan cheilostomes appear to have independently acquired opesiules (e.g. Cook 1965; Cook & Chimonides 1985; Cook & Bock 2001; Di Martino & Taylor 2012). It is supposed that in each group there is a tendency to form more complete calcified coverings of the frontal surface through development of the cryptocyst growing in a distal direction. In this case, the presence of parietal muscles attached to the frontal membrane is associated with the formation of the opesiules ( Ristedt 1991; Di Martino & Taylor 2012). Thus, opesiulate genera, including Coronellina View in CoL , must be considered as more derived. It follows that Mollia View in CoL , with no opesiules, is a less-derived genus than Coronellina View in CoL . We suggest that C. atlantica evolved from a form with opesiules in which parietal muscles were displaced distally. Accordingly, after losing their function the opesiules are in the process of disappearing ontogenetically, persisting as depressions. If this scenario is correct, then the new species described here, while lacking opesiules, is more appropriately placed in Coronellina View in CoL , as a more advanced form, and not in less-derived Mollia View in CoL . This also implies that, in contrast to the generally accepted evolutionary sequence from non-opesiulate to opesiulate forms, the reverse sequence is also possible.
Whatever the case, we assume that, taking into account the characters shared by Coronellina View in CoL and Mollia View in CoL (see above) both genera are closely related. The genus Coronellina View in CoL is currently included in the family Calescharidae View in CoL (see Gordon 2011; Bock & Hayward 2013a) and Mollia View in CoL in Microporidae View in CoL . Both families include genera with opesiules. Nevertheless, Calescharidae View in CoL has very large endozooidal, cleithral ovicells ( Cook & Bock 2001), whereas in Microporidae View in CoL ovicells are hyperstomial or immersed, acleithral, or absent ( Gordon 1984; Hayward & Ryland 1998; Ostrovsky 2013). The ovicell of Coronellina View in CoL has the same structure as Mollia View in CoL , so we believe that it belongs to the family Microporidae View in CoL .
Coronellina fagei View in CoL is present in the Mediterranean, but it was also reported from the Canary Islands ( Arístegui Ruiz 1984). However, this record must be checked, as the measurements given far exceed those of C. fagei View in CoL . This may indicate that the size of the species is widely variable or perhaps that C. fagei View in CoL is actually a species complex.
TABLE 1. Measurements (in mm) of Coronellina atlantica n. sp.
| Mean | SD | Minimum | Maximum | N | |
|---|---|---|---|---|---|
| Autozooid length | 0.435 | 0.0529 | 0.345 | 0.545 | 16 |
| Autozooid width | 0.283 | 0.0338 | 0.238 | 0.334 | 16 |
| Opesia length | 0.078 | 0.0057 | 0.065 | 0.086 | 16 |
| Opesia width | 0.125 | 0.0056 | 0.115 | 0.135 | 16 |
| Opesia ovicellate zooid length | 0.122 | 0.0123 | 0.107 | 0.136 | 7 |
| Opesia ovicellate zooid width | 0.153 | 0.0075 | 0.140 | 0.164 | 7 |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SubOrder |
Neocheilostomina |
|
InfraOrder |
Flustrina |
|
Family |
|
|
Genus |