Rhicnogryllus Chopard, 1925
|
publication ID |
https://doi.org/10.11646/zootaxa.4763.2.5 |
|
publication LSID |
urn:lsid:zoobank.org:pub:D179A5F1-C594-4B42-938E-3F8A2734BA84 |
|
DOI |
https://doi.org/10.5281/zenodo.3806446 |
|
persistent identifier |
https://treatment.plazi.org/id/039F4D5A-B963-FFB4-4BDE-FEA35CB309D0 |
|
treatment provided by |
Carolina |
|
scientific name |
Rhicnogryllus Chopard, 1925 |
| status |
|
Genus Rhicnogryllus Chopard, 1925
Chopard, 1925: 310; Chopard, 1968: 338; Desutter-Grandcolas, 1987: 236
Type species: Rhicnogryllus fascipes Chopard
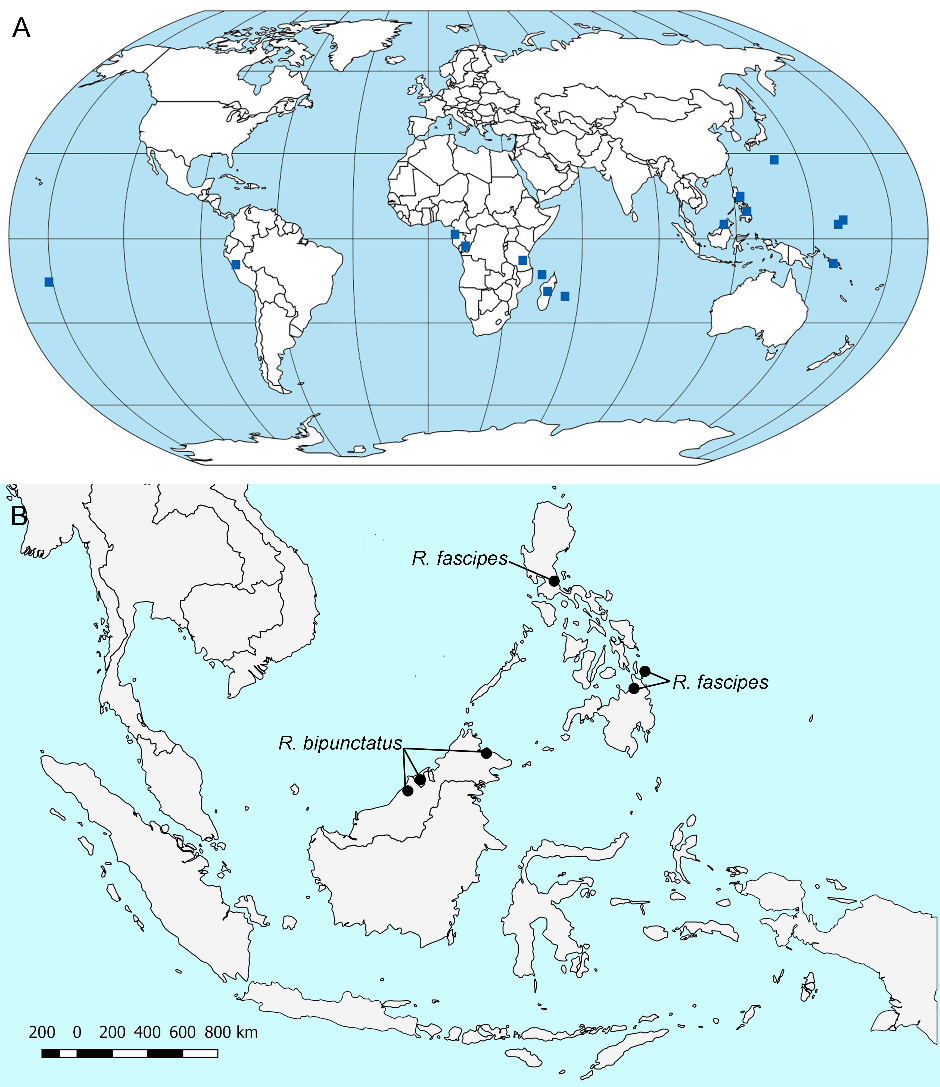
Distribution ( Fig. 1 View FIGURE 1 ). Southeast Asia: Philippines, Sarawak and Sabah (East Malaysia) and Brunei Darussalam ( Chopard, 1925, 1968; Ingrisch, 1987)
East Asia: Ogasawara ( Shiraki, 1930; Chopard, 1968; Ichikawa et al., 2000; Gorochov, 2018)
Pacific oceanic islands: Caroline Islands (Federated State of Micronesia), Solomon Islands, Tahiti ( French Polynesia) ( Saussure, 1878; Kirby, 1906; Hebard, 1926, 1933; Chopard, 1930, 1938, 1957, 1968; Willemse, 1951; Chopard, 1968)
Africa: Republic of Congo, Equatorial Guinea, Tanzania, Madagascar, Reunion Island and Comoros Islands ( Bolívar, 1910; Chopard, 1926, 1962, 1958, 1968; Desutter-Grandcolas, 1996)
South America: Peru ( Chopard, 1956, 1968; Aguilar, 1973)
Holocene Africa ( Chopard, 1936; Zeuner, 1939)
Description. Very small crickets, even among Trigonidiinae . Head, together with eyes, clearly wider than pronotum. Frontal rostrum flattened, only slightly wider than scapus; with a few long setae. Vertex flattened dorsally, with a few strong long setae. Antennal segments, including scapus, pubescent. Eye very large, protruding laterally, from dorsal view slightly elongated; in lateral view slightly hemispheric. Gena swollen, not pubescent. Maxillary palpi elongated; last three segments of about equal length; apical segment triangular, longer than wide, with apex truncated. Labial palpi with apical segment long and slightly swollen at the apex. Pronotum wider than long; with a few long setae but more pubescent along margins; sulci distinct or indistinct; anterior and posterior margins straight; dorsal disc flattened. Lateral lobe of pronotum longer than high, ventral margin straight or sinuous, anterior and posterior angles obtuse; anterior angle with numerous strong long setae, posterior margin lined with shorter setae. Without visible metanotal gland. Male and female tegmina in lateral view appears rounded, not pubescent; reaching abdominal apex, but not covering epiproct; without stridulatory apparatus in males; apex somewhat truncated. Tegminal dorsal field with 7 longitudinal, elevated and parallel veins, lateral field with 3 or 4 less-elevated and slightly sinuous veins; without cross-vein. Hind wing absent. Legs generally pubescent and with long setae. Fore tibia with both tympana absent. Tarsal middle segment with prominent adhesive pad. Hind tibia with three inner and three outer subapical spurs; two inner and two to three outer apical spurs, inner ones distinctly longer than outer ones.
Female: Not different morphologically as the males, including tegmina. Ovipositor short, barely reaching apex of cercus; basal half straight and apical half slightly curved dorsad; ventral and dorsal margins usually dentated; ventral valve surpassing usually dorsal valve, apex acute.
Colouration: Highly variable, but often with contrasting colour patterns. Exhibit sexual dimorphism in some species—typically with females having darker colouration (e.g., Rhicnogryllus fascipes Chopard, 1925 )—but not others (e.g., Rhicnogryllus bipunctatus Ingrisch, 1987 ). Hind femur usually pale colour with two black bands, one in the middle, and another either before or after middle.
Comparison with similar genera. Rhicnogryllus (sensu R. bipunctatus ) has similar genitalia morphology as Trigonidium and Svistella Gorochov, 1987 but differs from Trigonidium by virga long (usually shorter than rachis in Trigonidium ). Rhicnogryllus also differs from Trigonidium ( Paratrigonidium Brunner von Wattenwyl, 1893 ) and Svistella by absence of stridulatory organs in males (see Gorochov, 1987; Tan & Robillard, 2012; Lu et al., 2018; Tan et al., 2019a).
Discussion. Chopard (1925, 1930) specified that the main characteristics that define this genus from closely related ones, such as Trigonidium and Metioche is the strongly elevated veins on the tegmina, but species from different parts of the world can share such characters. However, the strongly elevated veins on the tegmina in Rhicnogryllus from different parts of the world are probably an outcome of convergence rather than relatedness. We have already observed that the genitalia of R. bipunctatus and our new species Rhicnogryllus ? paetensis n. sp. differ drastically from an African representative, Rhicnogryllus lepidus Chopard, 1962 ( Desutter-Grandcolas, 1996).
The cosmopolitan distribution of this genus of small crickets that do not have apparent ability to fly and likely to have specialised niche, as well as vastly different genitalia morphology, strongly suggest that Rhicnogryllus is artificial and that species from different biogeographic regions may belong to different genera. Our hypothesis is that the current Rhicnogryllus is probably a genus endemic to Southeast Asia and/or Pacific oceanic islands and East Asia (since the type species is from the Philippines). Species from Africa and South America probably belong to different genera. Nonetheless, in the current state of Trigonidiinae taxonomy, introducing new genera may only add more confusion when the other taxa are not revised and examined.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Trigonidiinae |
|
Tribe |
Trigonidiini |
Rhicnogryllus Chopard, 1925
| Tan, Ming Kai, Baroga-Barbecho, Jessica B., Japir, Razy, Chung, Arthur Y. C., Wahab, Rodzay Bin Haji Abdul & Yap, Sheryl A. 2020 |
Rhicnogryllus
| Chopard, 1925: 310 |
| Chopard, 1968: 338 |
| Desutter-Grandcolas, 1987 : 236 |