Milyeringa veritas Whitley, 1945
|
publication ID |
https://doi.org/10.11646/zootaxa.3616.2.3 |
|
publication LSID |
lsid:zoobank.org:pub:D6547090-2354-428C-B7EA-59C8B3795394 |
|
DOI |
https://doi.org/10.5281/zenodo.5613148 |
|
persistent identifier |
https://treatment.plazi.org/id/03A087D5-FFB2-D96D-F7C3-6A7A0871FE76 |
|
treatment provided by |
Plazi |
|
scientific name |
Milyeringa veritas Whitley, 1945 |
| status |
|
Milyeringa veritas Whitley, 1945 View in CoL
Cave gudgeon
Milyeringa veritas Whitley, 1945: 36 –37 (Milyering, Yardie, 20 miles south-west of Vlamingh Head, North-west Cape, Western Australia).
Milyeringa brooksi Chakrabarty, 2010: 22 –25 (Pilgonaman Well, North West Cape).
Diagnosis. A Milyeringa with small first dorsal fin of II–IV spines, usually III; 6–8 segmented second dorsal fin rays; I,6–8 anal fin rays; pectoral fin rays 11–14; pelvic fin rays I,4; 16–17 segmented caudal fin rays; caudal and pectoral fin rays all unbranched; body fully scaled with scales usually extending forward onto predorsal, 22–29 lateral scales, head short with reduced numbers of rows of sensory papillae on the head and body, posteroventral margin of preopercle forming expanded bony flange; vertebrae 10–12+14–15 ( 24–26 in total), two pre-anal pterygiophores, one epural. Found in caves and wells of North-West Cape, Western Australia.
Material examined. WESTERN AUSTRALIA: WAM P.2913, holotype of Milyeringa veritas , 39 mm SL female, Yardie Station; WAM P.28330-001, holotype of Milyeringa brooksi , 36.5 mm SL female, Pilgonaman Well, North-west Cape, M. Newton, 8 July 1984; WAM P.29242-001, paratypes of Milyeringa brooksi , 2(36–38.5), Exmouth, B. Vine and party, 19 May 1985; WAM P.28262-001, 8(12.5–40.5), Milyering Well, North-west Cape; WAM P.33161-001, 2(34–36), Kubura Well, Cape Range, W.F. Humphreys, 1 July 1993; WAM P.33157-001, 2(22–24), Five Mile Well, Cape Range, B. Vine, 15 July 1989; WAM P.33168-001, 1(22.5), Exmouth bore field Water Corporation bore 18, Cape Range, Kinhill, 1 April 2001; WAM P.33163-001, 1(13.5), Ampolex site D, Cape Range, R.D. Brooks, 12 November 1995; WAM P.33160-001, 3(31–37), Kubura Well, Cape Range, W.F. Humphreys, 12 August 1993; WAM P.33158-001, 1(32.5), Cape Range, W.F. Humphreys, 15 July 1993; WAM P.33159-001, 2(25–30.5), Kubura Well, Cape Range, W.F. Humphreys, 26 May 1993; WAM P.33164-001, 1(13), Cape Range, A. Poole and S. Eberhard, 22 September 1997; AMS I.25504-001, 1(31), cleared and stained, well 3 km S of Mangrove Bay, Cape Range National Park, D. Hoese and D. Rennis, 13 September 1985.
X-rayed specimens: F03762, 1, North West Cape, West Australia, 21° 47' S 114° 10' E; F05491, 3, township rockhole, Exmouth, West Australia, 21° 56' S 114° 7' E’); F05492, 3, rockhole 6 km north of Yardie Creek, West Australia, 22° 20' S 113° 51' E.
Description. Based on 23 specimens, 12.5–40.5 mm SL. Counts and morphometrics of the holotype of Milyeringa veritas indicated by asterisk.
First dorsal spines II–IV*, modally III; second dorsal rays 6–8*, modally 8; anal rays I,6–8, modally I,7* pectoral rays 11–14, modally 13; pelvic rays I,4; segmented caudal rays 16–17*, in 8/8 (in 4) or 9/8* pattern (16), all fin rays unbranched; lateral scale count 22–29*, modally 26; transverse scales backward 9–12, modally 10 ( 11 in holotype); predorsal scale count 0–16*, modally 12; vertebrae 10+14 (1), 11+14 (1), 11+15 (4), 12+14 (1); dorsal pterygiophore pattern 4–221 (in 1); 1 epural; 2 anal pterygiophores anterior to first haemal spine; last haemal spine broadened or forked (with variable amount of sheet of bone between forks).
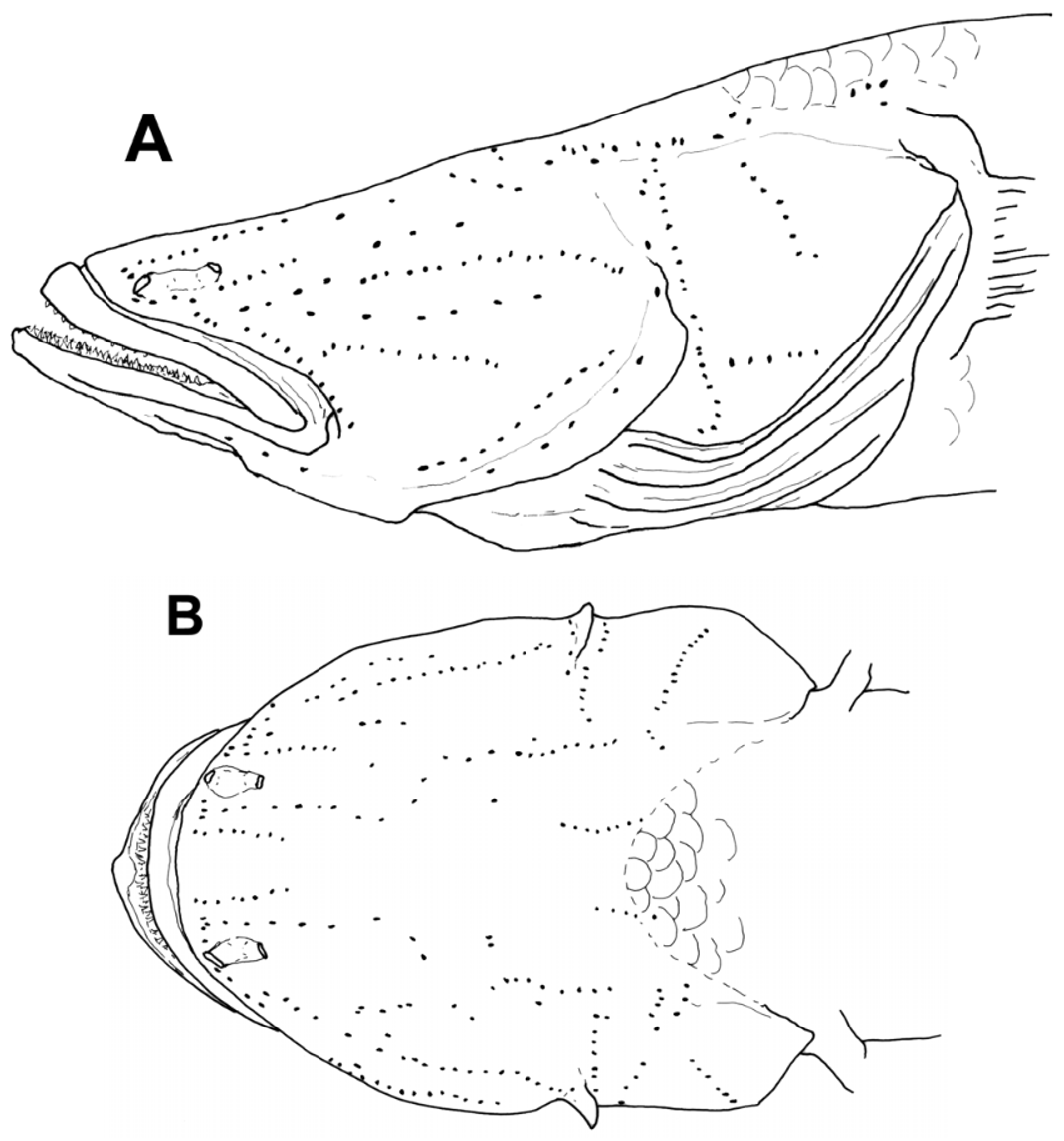
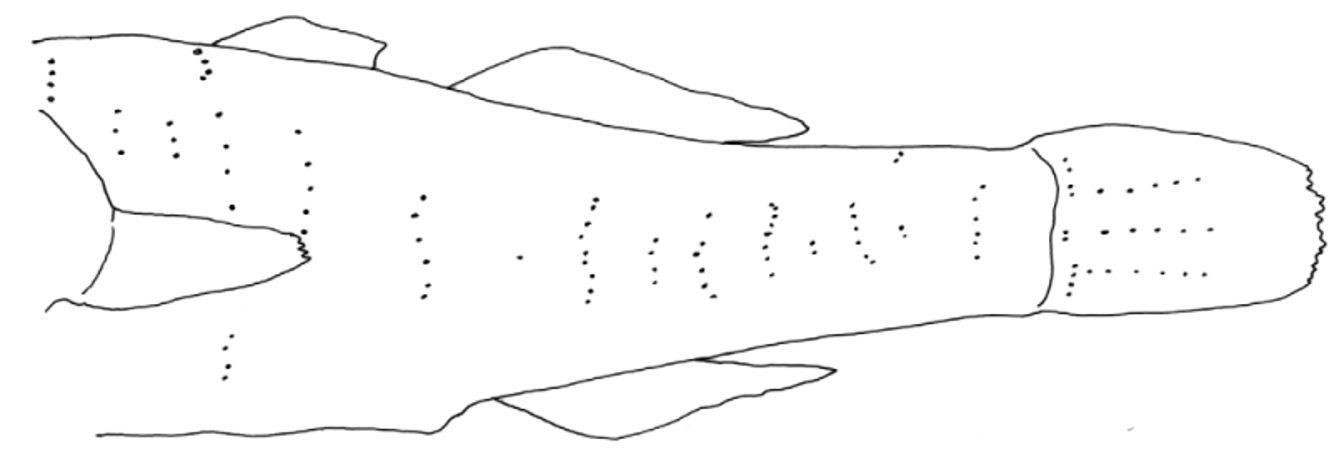
Body moderate, somewhat rounded anteriorly, compressed posteriorly; body depth at anal fin origin 12.3–20.0% of SL. Caudal peduncle length 20.0–27.7% of SL. Caudal peduncle depth 7.7–11.8% of SL. Head rounded to depressed, larger specimens (over 30 mm SL) with head much wider than deep at preopercular margin, head length 36.9–42.3% of SL; head depth at posterior preopercular margin 35.4–53.5% of HL; width at posterior preopercular margin 49.5–73.7% of HL. Mouth large, terminal and oblique, chin tip anteriormost, jaws forming an angle of about 35º with body axis. Upper jaw length 27.1–43.8% of HL; inner margin of lips smooth; lower lip fused to chin anteriorly, side of lip free; chin flat with no mental frenum. Anterior naris in short tube at edge of or just above upper lip; posterior naris oval to flattened oval, with low rim (or very short tube), occasionally anterior rim slightly produced as low curved flap; nares joined by thin “tube” over nasal rosette. Eyes absent. Snout area broad, flattened, tilted upward slightly, rounded to nearly square anteriorly in dorsal view; snout slightly pointed in lateral view mostly in small specimens, length of snout tip to upper edge of the preopercular margin 65.5–72.9% of HL. Opercle length (upper edge of preopercle to upper margin of opercle) 27.8–39.8% of HL. Preopercular margin bony and broadened into flange posteroventrally, extending from about halfway down posterior margin nearly to rictus; flange present even in small specimens (e.g. 15 mm SL), gently curved outward in some specimens (may not be size-related), flange may be angular or with indentation near corner. Gill opening wide and somewhat variable in anterior extent, reaching forward from just before preopercular rear margin (as in holotype) to halfway between preopercular rear margin and rictus. Tongue large, tip blunt. Teeth in both jaws small, evenly sized, conical and pointed; in two to three rows; teeth of inner rows larger, pointing inward in larger specimens and more upright in smaller. Headpores absent. Sensory papillae conspicuous fleshy bumps, in longitudinal pattern; papillae with thin narrow flap each; thin flaps usually present only on well-preserved specimens ( Fig. 9 View FIGURE 9 ). Sensory papillae on body on body in variable numbers of irregular vertical (transverse) rows, often interspersed with single papillae and few papillae along hypural crease; papillae on body sometimes with broad to thin narrow flaps (uncertain if absence actual or due to damage); three longitudinal rows of papillae on caudal fin ( Fig. 10 View FIGURE 10 ).
Body covered with small cycloid scales, reaching forward onto head above level of opercle or above rear preopercular margin. Side of head naked. Prepelvic region naked (usually) or with patch of cycloid scales just before pelvic fin bases. Pectoral fin base naked. Belly covered with cycloid scales.
First dorsal fin greatly reduced, roughly triangular, with gap of about three or four scales before the second dorsal fin origin. Posteriormost second dorsal and anal fins short-based, rays taller than first dorsal fin; posterior rays slightly longer than anterior rays but not greatly so, posterior rays falling well short of caudal fin base. Second dorsal fin with all elements segmented. Anal fin usually with spine and segmented rays (three specimens with all elements segmented). Pectoral fin small, slender, pointed, central rays longest, 12.3–28.9% of SL; all rays unbranched. Pelvic fin length 7.7–26.9% of SL; fins small, very slender and pointed, fins extending less than half the distance to anus. Caudal fin oval, with central rays elongate, often greatly so (elongate rays often damaged or broken in available specimens); caudal fin length 18.8–39.2% of SL.
Live coloration. Photos of living fish are shown in Allen (1982), Merrick and Schmida (1984), Young (1986) and Allen et al. (2002) ( Fig. 11 View FIGURE 11 ). Fish white to translucent white posteriorly, with transparent fins, and red gills and pinkish colour from abdominal organs showing through opercle and body wall.
Coloration in alcohol. Whitish, with transparent fin membranes. Specimens often with very fine scattered blackish to dark brown melanophores over dorsal surface of cranium and frontals (i.e. would-be interorbital space).
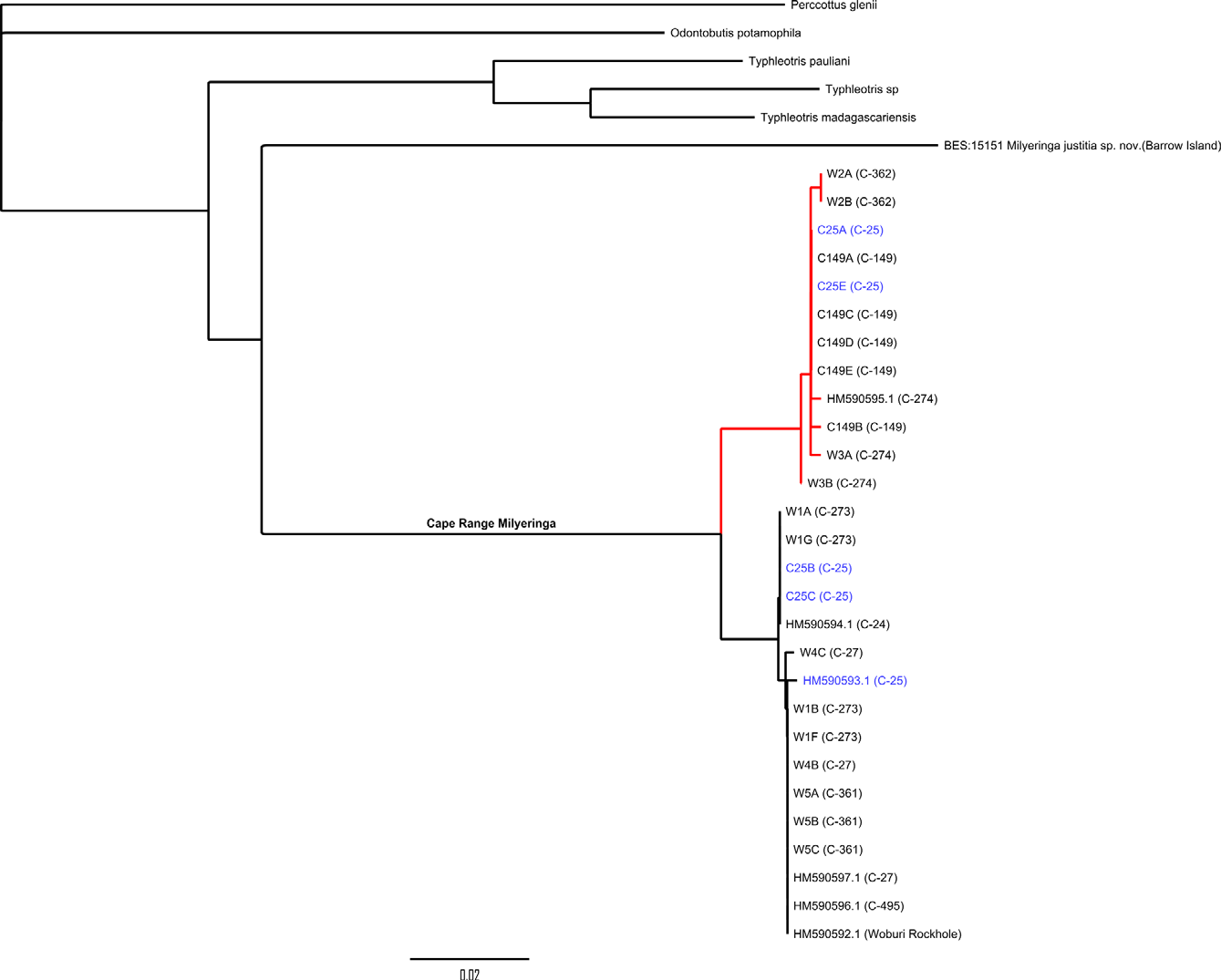
Results of genotyping. The branch of the recovered COI gene tree representing mainland (Cape Range peninsula) Milyeringa closely resembles Chakrabarty’s (2010) tree in topology and genetic distances within and between two major clades ( Fig. 3 View FIGURE 3 ) though greater substructure is evident. Most significantly, however, we found both major mitochondrial lineages co-occurring at C-25, Kudamurra Well (samples in blue in the NJ tree, Fig. 3 View FIGURE 3 ). This population shows no concordant allozyme differentiation between individuals of ‘ veritas ’ haplotype and those of ‘ brooksi ’ haplotype (Adams and Humphreys 1993), a finding consistent with the presence of free gene flow and a single species, Milyeringa veritas .
Distribution. Known from Cape Range peninsula, Western Australia.
Ecology. Milyeringa veritas is listed as vulnerable under the Environment Protection and Biodiversity Conservation Act 1999 (Commonwealth) and as endangered under Schedule One of the Wildlife Conservation Act 1950 (Western Australia) owing to its small geographic distribution, low populations and vulnerability of its habitat. Two locations in which the species occurs (Bundera Sinkhole and Camerons Cave) are also listed as threatened communities (by the Western Australian Government’s Department of Environment and Conservation). Its distribution lies within the Ningaloo Coast World Heritage Area. The conservation status is discussed by Romero and Vanselow (2000).
The sparse knowledge of the biology of Milyeringa was summarised by Humphreys (2001a). Milyeringa veritas inhabits the coastal groundwaters of the carbonate karst of the Cape Range peninsula that projects onto the North West Shelf of Australia. It occurs on the coastal plain and foothills of the peninsula between 0.4 km and 4.0 km of the coast. Closer to the coast is an anchialine system, characteristically with marked hydrogeochemical stratification with depth (Seymour et al. 2007), but grading to freshwater inland. The fish occupy a wide range of salinity, from freshwater inland to fully marine in the depths of the anchialine system (Humphreys 2001a). Otolith microchemistry indicates that individuals may inhabit waters of widely different salinity through their lives (Humphreys et al. 2006). They seem to be opportunistic feeders ingesting prey of epigean origin as well as obligate groundwater species (Humphreys & Feinberg 1995).
Anchialine habitats of the type inhabited by Milyeringa are noteworthy for their very diverse stygiobiont crustacean fauna, comprising a characteristic assemblage of higher taxa, the structure of which is highly predictable, frequently extending to the generic composition (Wagner 1994), however far apart in the world they occur (Jaume et al. 2001); most of their members represent biogeographic and/or phylogenetic relicts (Iliffe 1992). As such, the anchialine fauna of the Cape Range peninsula has an assemblage comparable to anchialine systems of the North Atlantic comprising, inter alia, the higher taxa Remipedia, Thermosbaenacea , Atyidae (Decapoda) , Hadziidae (Amphipoda) , Cirolanidae (Isopoda) , Thaumatocypridae (Ostracoda), and the copepod taxa Calanoida ( Epacteriscidae , Ridgewayiidae and Pseudocyclopiidae ), Misophrioida (Speleophriidae) and Cyclopoida (Halicyclopinae) . Milyeringa veritas is sympatric with the groundwater synbranchid eel Ophisternon candidum (Mees, 1962) .
Remarks. Milyeringa brooksi was described by Chakrabarty (2010), who separated his species from M. veritas mainly by its having 10–12 vertical lines of sensory papillae along the body and by “molecular synapomorphies” (which refer to changes in nucleotide position in three genes: ND2, CytB and COI). Chakrabarty also considered that M. brooksi was a smaller fish than M. veritas (the largest specimen given as 38.33 mm SL versus 52.6 in M. veritas ), had a tubular posterior nostril with a skin flap (versus a simple round nostril in M. veritas ) and had conspicuous papillae on the dorsal surface of the head (versus a “variable condition” in M. veritas ). The nostril size and shape in the holotype of M. brooksi and the two WAM paratypes are basically the same as in M. veritas . We cannot distinguish his specimens as a separate species from M. veritas .
We observed features in Milyeringa specimens that were missed by Chakrabarty (2010). He indicated that he had 16 specimens of M. veritas (sizes given as 30.7–52.6 mm SL) to compare with his seven specimens of M. brooksi . He apparently did not observe the vertical rows of sensory papillae along the side of the body in M. veritas which are present in specimens of all sizes and locations. He also describes the head of M. brooksi as being naked, although scales can be observed on the holotype’s predorsal region, extending forward to above the preopercular margin.
Chakrabarty’s 2010 DNA sequence data for Milyeringa were derived from eight specimens from seven populations. The maximum genetic distance found between the two major mitochondrial lineages that represent M. veritas and M. brooksi was 3.2%. The small sample size and low genetic distance (in comparison with genetic distances found among other eleotrid species; e.g. Stevens & Hicks 2009, Thacker 2009) are themselves problematic but, as highlighted in recent discussions of the use of barcoding (e.g. Lohse 2009; Petit & Excoffier 2009), the use of markers from a single uniparentally inherited genome for species delimitation is risky and the inference of a species tree from what is effectively a single gene tree, inadvisable. Multilocus assignment methods, using unlinked markers, have considerably more power in this respect and can also inform on population admixtures.
Humphreys and Adams (1991) and Adams and Humphreys (1993) subjected seven populations of Milyeringa veritas (n = 24 & 29, respectively) to comprehensive allozyme analyses at 43 loci and demonstrated that, although allele frequencies and distributions indicated significant population sub-structuring on the peninsula, overall levels of differentiation and the pattern of allele distribution were consistent with the presence of a single biological species on Cape Range peninsula. The mitochondrial data presented here affirm the view established in the 1990s and provide additional evidence for the distinctiveness of the species from Barrow Island.
Conclusions. Cape Range peninsula and Barrow Island are home to a distinctive and unique stygofauna including cave-dwelling fishes. The description of a new and highly divergent Milyeringa from within the range of an already restricted taxon, adds significantly to the regional fauna with matching conservation implications, in the face of increasing and broad-scale mineral exploration. We recognise that further systematic work may be required to understand the evolutionary heritage of Milyeringa species.
Acknowledgements
Our thanks to Peter Austin, Australian Minerals Research Centre, CSIRO and the late Peter Blias of SAMA who created microtomography scans and reconstructions of M. justitia and M. veritas . Our thanks also to Doug Hoese and Tony Gill for their generous sharing of data and valuable discussions on Milyeringa osteology. Thanks to Michael Hammer for his discussions with HKL on some points of genetic data. Specimen collection on Barrow Island was facilitated by Western Australian Petroleum Pty Ltd, and Chevron Australia.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |